Anterior-posterior pattern formation in ciliates
- PMID: 35075744
- PMCID: PMC9309198
- DOI: 10.1111/jeu.12890
Anterior-posterior pattern formation in ciliates
Abstract
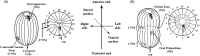
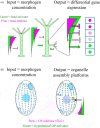
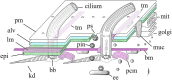
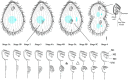
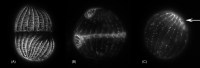
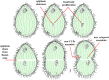
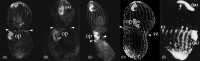
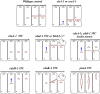
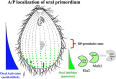
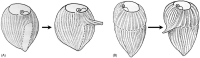
As single cells, ciliates build, duplicate, and even regenerate complex cortical patterns by largely unknown mechanisms that precisely position organelles along two cell-wide axes: anterior-posterior and circumferential (left-right). We review our current understanding of intracellular patterning along the anterior-posterior axis in ciliates, with emphasis on how the new pattern emerges during cell division. We focus on the recent progress at the molecular level that has been driven by the discovery of genes whose mutations cause organelle positioning defects in the model ciliate Tetrahymena thermophila. These investigations have revealed a network of highly conserved kinases that are confined to either anterior or posterior domains in the cell cortex. These pattern-regulating kinases create zones of cortical inhibition that by exclusion determine the precise placement of organelles. We discuss observations and models derived from classical microsurgical experiments in large ciliates (including Stentor) and interpret them in light of recent molecular findings in Tetrahymena. In particular, we address the involvement of intracellular gradients as vehicles for positioning organelles along the anterior-posterior axis.
Keywords: cell division; ciliates; cortical; gradients; kinase; morphogens; patterning; tetrahymena.
© 2022 The Authors. Journal of Eukaryotic Microbiology published by Wiley Periodicals LLC on behalf of International Society of Protistologists.
Figures


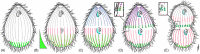


References
-
- Albrecht‐Buehler, G. (1977) Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell, 12, 333–339. - PubMed
-
- Allen, R.D. (1967) Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. The Journal of Protozoology, 14, 553–565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

