Behavioural and molecular characterisation of the Dlg2 haploinsufficiency rat model of genetic risk for psychiatric disorder
- PMID: 35075790
- PMCID: PMC9393932
- DOI: 10.1111/gbb.12797
Behavioural and molecular characterisation of the Dlg2 haploinsufficiency rat model of genetic risk for psychiatric disorder
Abstract
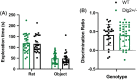
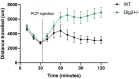
Genetic studies implicate disruption to the DLG2 gene in copy number variants as increasing risk for schizophrenia, autism spectrum disorders and intellectual disability. To investigate psychiatric endophenotypes associated with DLG2 haploinsufficiency (and concomitant PSD-93 protein reduction) a novel clinically relevant Dlg2+/- rat was assessed for abnormalities in anxiety, sensorimotor gating, hedonic reactions, social behaviour, and locomotor response to the N-Methyl-D-aspartic acid receptor antagonist phencyclidine. Dlg gene and protein expression were also investigated to assess model validity. Reductions in PSD-93 messenger RNA and protein were observed in the absence of compensation by other related genes or proteins. Behaviourally Dlg2+/- rats show a potentiated locomotor response to phencyclidine, as is typical of psychotic disorder models, in the absence of deficits in the other behavioural phenotypes assessed here. This shows that the behavioural effects of Dlg2 haploinsufficiency may specifically relate to psychosis vulnerability but are subtle, and partially dissimilar to behavioural deficits previously reported in Dlg2+/- mouse models demonstrating issues surrounding the comparison of models with different aetiology and species. Intact performance on many of the behavioural domains assessed here, such as anxiety and reward processing, will remove these as confounds when continuing investigation into this model using more complex cognitive tasks.
Keywords: DLG2; PCP locomotion; animal models; autism spectrum disorder; schizophrenia; sensorimotor gating; synaptic plasticity.
© 2022 The Authors. Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm there are no conflicts of interest to declare.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

