Rational design of anti-inflammatory lipid nanoparticles for mRNA delivery
- PMID: 35076171
- PMCID: PMC10155289
- DOI: 10.1002/jbm.a.37356
Rational design of anti-inflammatory lipid nanoparticles for mRNA delivery
Abstract
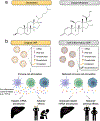
Lipid nanoparticles (LNPs) play a crucial role in delivering messenger RNA (mRNA) therapeutics for clinical applications, including COVID-19 mRNA vaccines. While mRNA can be chemically modified to become immune-silent and increase protein expression, LNPs can still trigger innate immune responses and cause inflammation-related adverse effects. Inflammation can in turn suppress mRNA translation and reduce the therapeutic effect. Dexamethasone (Dex) is a widely used anti-inflammatory corticosteroid medication that is structurally similar to cholesterol, a key component of LNPs. Here, we developed LNP formulations with anti-inflammatory properties by partially substituting cholesterol with Dex as a means to reduce inflammation. We demonstrated that Dex-incorporated LNPs effectively abrogated the induction of tumor necrosis factor alpha (TNF-ɑ) in vitro and significantly reduced its expression in vivo. Reduction of inflammation using this strategy improved in vivo mRNA expression in mice by 1.5-fold. Thus, we envision that our Dex-incorporated LNPs could potentially be used to broadly to reduce the inflammatory responses of LNPs and enhance protein expression of a range of mRNA therapeutics.
Keywords: anti-inflammation; dexamethasone; gene delivery; lipid nanoparticles; mRNA.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest.
Figures


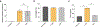
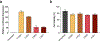

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

