Identification of a STIM1 Splicing Variant that Promotes Glioblastoma Growth
- PMID: 35076181
- PMCID: PMC9008427
- DOI: 10.1002/advs.202103940
Identification of a STIM1 Splicing Variant that Promotes Glioblastoma Growth
Abstract
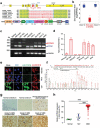
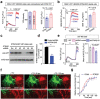
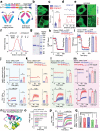
Deregulated store-operated calcium entry (SOCE) mediated by aberrant STIM1-ORAI1 signaling is closely implicated in cancer initiation and progression. Here the authors report the identification of an alternatively spliced variant of STIM1, designated STIM1β, that harbors an extra exon to encode 31 additional amino acids in the cytoplasmic domain. STIM1β, highly conserved in mammals, is aberrantly upregulated in glioma tissues to perturb Ca2+ signaling. At the molecular level, the 31-residue insertion destabilizes STIM1β by perturbing its cytosolic inhibitory domain and accelerating its activation kinetics to efficiently engage and gate ORAI calcium channels. Functionally, STIM1β depletion affects SOCE in glioblastoma cells, suppresses tumor cell proliferation and growth both in vitro and in vivo. Collectively, their study establishes a splicing variant-specific tumor-promoting role of STIM1β that can be potentially targeted for glioblastoma intervention.
Keywords: STIM1; calcium signaling; cell signaling; glioblastoma; splicing.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition.Sci Rep. 2016 Feb 26;6:22142. doi: 10.1038/srep22142. Sci Rep. 2016. PMID: 26917047 Free PMC article.
-
The STIM-Orai Pathway: Conformational Coupling Between STIM and Orai in the Activation of Store-Operated Ca2+ Entry.Adv Exp Med Biol. 2017;993:83-98. doi: 10.1007/978-3-319-57732-6_5. Adv Exp Med Biol. 2017. PMID: 28900910 Free PMC article. Review.
-
Ca2+/Calmodulin Binding to STIM1 Hydrophobic Residues Facilitates Slow Ca2+-Dependent Inactivation of the Orai1 Channel.Cell Physiol Biochem. 2020 Mar 17;54(2):252-270. doi: 10.33594/000000218. Cell Physiol Biochem. 2020. PMID: 32176842
-
Filamin A Modulates Store-Operated Ca2+ Entry by Regulating STIM1 (Stromal Interaction Molecule 1)-Orai1 Association in Human Platelets.Arterioscler Thromb Vasc Biol. 2018 Feb;38(2):386-397. doi: 10.1161/ATVBAHA.117.310139. Epub 2017 Dec 28. Arterioscler Thromb Vasc Biol. 2018. PMID: 29284605
-
Proteins Interacting with STIM1 and Store-Operated Ca2+ Entry.Prog Mol Subcell Biol. 2021;59:51-97. doi: 10.1007/978-3-030-67696-4_4. Prog Mol Subcell Biol. 2021. PMID: 34050862 Review.
Cited by
-
STIM1 functions as a proton sensor to coordinate cytosolic pH with store-operated calcium entry.J Biol Chem. 2024 Dec;300(12):107924. doi: 10.1016/j.jbc.2024.107924. Epub 2024 Oct 23. J Biol Chem. 2024. PMID: 39454952 Free PMC article.
-
Suppressing ERp57 diminishes osteoclast activity and ameliorates ovariectomy-induced bone loss via the intervention in calcium oscillation and the calmodulin/calcineurin/Nfatc1 pathway.Heliyon. 2024 Jul 27;10(15):e35374. doi: 10.1016/j.heliyon.2024.e35374. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170388 Free PMC article.
-
Modification of BCLX pre-mRNA splicing has antitumor efficacy alone or in combination with radiotherapy in human glioblastoma cells.Cell Death Dis. 2024 Feb 21;15(2):160. doi: 10.1038/s41419-024-06507-x. Cell Death Dis. 2024. PMID: 38383492 Free PMC article.
-
STIM1 in tumor cell death: angel or devil?Cell Death Discov. 2023 Nov 6;9(1):408. doi: 10.1038/s41420-023-01703-8. Cell Death Discov. 2023. PMID: 37932320 Free PMC article. Review.
-
Role of STIM1 in the Regulation of Cardiac Energy Substrate Preference.Int J Mol Sci. 2023 Aug 25;24(17):13188. doi: 10.3390/ijms241713188. Int J Mol Sci. 2023. PMID: 37685995 Free PMC article. Review.
References
-
- a) Prakriya M., Lewis R. S., Physiol. Rev. 2015, 95, 1383. - PMC - PubMed
- b) Hogan P. G., Lewis R. S., Rao A., Annu. Rev. Immunol. 2010, 28, 491. - PMC - PubMed
- c) Soboloff J., Rothberg B. S., Madesh M., Gill D. L., Nat. Rev. Mol. Cell Biol. 2012, 13, 549. - PMC - PubMed
- d) Nguyen N. T., Han W., Cao W. M., Wang Y., Wen S., Huang Y., Li M., Du L., Zhou Y., Compr. Physiol. 2018, 8, 981. - PubMed
-
- a) Rosado J. A., Diez R., Smani T., Jardin I., Front. Pharmacol. 2015, 6, 325. - PMC - PubMed
- b) Darbellay B., Arnaudeau S., Bader C. R., Konig S., Bernheim L., J. Cell Biol. 2011, 194, 335. - PMC - PubMed
- c) Miederer A. M., Alansary D., Schwar G., Lee P. H., Jung M., Helms V., Niemeyer B. A., Nat. Commun. 2015, 6, 6899. - PMC - PubMed
- d) Rana A., Yen M., Sadaghiani A. M., Malmersjo S., Park C. Y., Dolmetsch R. E., Lewis R. S., J. Cell Biol. 2015, 209, 653. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
