Immune Maturation Effects on Viral Neutralization and Avidity of Hyperimmunized Equine Anti-SARS-CoV-2 Sera
- PMID: 35076465
- PMCID: PMC8788445
- DOI: 10.3390/antib11010003
Immune Maturation Effects on Viral Neutralization and Avidity of Hyperimmunized Equine Anti-SARS-CoV-2 Sera
Abstract
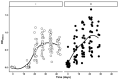
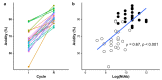
Mass-vaccination against COVID-19 is still a distant goal for most low-to-middle income countries. The experience gained through decades producing polyclonal immunotherapeutics (such as antivenoms) in many of those countries is being redirected to develop similar products able to neutralize SARS-CoV-2 infection. In this study we analyzed the biological activity (viral neutralization or NtAb) and immunochemical properties of hyperimmune horses' sera (HHS) obtained during initial immunization (I) and posterior re-immunization (R) cycles using the RBD domain of the SARS-CoV-2 spike protein as antigen. HHS at the end of the R cycle showed higher NtAb titers when compared to those after the I cycle (35,585 vs. 7000 mean NtAb, respectively). Moreover, this increase paralleled an increase in avidity (95.2% to 65.2% mean avidity units, respectively). The results presented herein are relevant for manufacturers of these therapeutic tools against COVID-19.
Keywords: COVID-19; anti-SARS-CoV-2; hyperimmune equine; hyperimmunization; immune maturation; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lopardo G., Belloso W.H., Nannini E., Colonna M., Sanguineti S., Zylberman V., Muñoz L., Dobarro M., Lebersztein G., Farina J., et al. RBD-specific polyclonal F(ab’)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021;34:100843. doi: 10.1016/j.eclinm.2021.100843. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

