Molecular regulation of autophagosome formation
- PMID: 35076688
- PMCID: PMC9022990
- DOI: 10.1042/BST20210819
Molecular regulation of autophagosome formation
Abstract
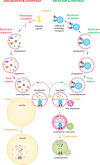
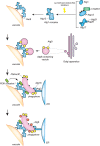
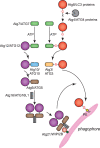
Macroautophagy, hereafter autophagy, is a degradative process conserved among eukaryotes, which is essential to maintain cellular homeostasis. Defects in autophagy lead to numerous human diseases, including various types of cancer and neurodegenerative disorders. The hallmark of autophagy is the de novo formation of autophagosomes, which are double-membrane vesicles that sequester and deliver cytoplasmic materials to lysosomes/vacuoles for degradation. The mechanism of autophagosome biogenesis entered a molecular era with the identification of autophagy-related (ATG) proteins. Although there are many unanswered questions and aspects that have raised some controversies, enormous advances have been done in our understanding of the process of autophagy in recent years. In this review, we describe the current knowledge about the molecular regulation of autophagosome formation, with a particular focus on budding yeast and mammalian cells.
Keywords: ATG proteins; autophagy; degradation; lysosomes; phagophore; sequestration.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Phagophore closure, autophagosome maturation and autophagosome fusion during macroautophagy in the yeast Saccharomyces cerevisiae.FEBS Lett. 2024 Jan;598(1):73-83. doi: 10.1002/1873-3468.14720. Epub 2023 Aug 23. FEBS Lett. 2024. PMID: 37585559 Review.
-
Expanding the view of the molecular mechanisms of autophagy pathway.J Cell Physiol. 2022 Aug;237(8):3257-3277. doi: 10.1002/jcp.30819. Epub 2022 Jul 5. J Cell Physiol. 2022. PMID: 35791448 Review.
-
Membrane Contact Sites in Autophagy.Cells. 2022 Nov 28;11(23):3813. doi: 10.3390/cells11233813. Cells. 2022. PMID: 36497073 Free PMC article. Review.
-
Autophagy pathway: Cellular and molecular mechanisms.Autophagy. 2018;14(2):207-215. doi: 10.1080/15548627.2017.1378838. Epub 2017 Dec 31. Autophagy. 2018. PMID: 28933638 Free PMC article. Review.
-
A nuclear membrane-derived structure associated with Atg8 is involved in the sequestration of selective cargo, the Cvt complex, during autophagosome formation in yeast.Autophagy. 2019 Mar;15(3):423-437. doi: 10.1080/15548627.2018.1525475. Epub 2018 Oct 11. Autophagy. 2019. PMID: 30238844 Free PMC article.
Cited by
-
The Role of mTORC1 Pathway and Autophagy in Resistance to Platinum-Based Chemotherapeutics.Int J Mol Sci. 2023 Jun 26;24(13):10651. doi: 10.3390/ijms241310651. Int J Mol Sci. 2023. PMID: 37445831 Free PMC article. Review.
-
TRIM72 Alleviates Muscle Inflammation in mdx Mice via Promoting Mitophagy-Mediated NLRP3 Inflammasome Inactivation.Oxid Med Cell Longev. 2023 Jan 18;2023:8408574. doi: 10.1155/2023/8408574. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36713032 Free PMC article.
-
Oxytocin Modulates Osteogenic Commitment in Human Adipose-Derived Stem Cells.Int J Mol Sci. 2023 Jun 28;24(13):10813. doi: 10.3390/ijms241310813. Int J Mol Sci. 2023. PMID: 37445991 Free PMC article.
-
The deubiquitinase USP45 inhibits autophagy through actin regulation by Coronin 1B.J Cell Biol. 2025 May 5;224(5):e202407014. doi: 10.1083/jcb.202407014. Epub 2025 Mar 11. J Cell Biol. 2025. PMID: 40067150 Free PMC article.
-
ATG10-dependent autophagy is required for DDX10 to regulate cell proliferation, apoptosis and stemness in colorectal cancer.J Cancer Res Clin Oncol. 2024 Aug 7;150(8):386. doi: 10.1007/s00432-024-05910-3. J Cancer Res Clin Oncol. 2024. PMID: 39110225 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

