Development and Validation of a Treatment Benefit Index to Identify Hospitalized Patients With COVID-19 Who May Benefit From Convalescent Plasma
- PMID: 35076698
- PMCID: PMC8790670
- DOI: 10.1001/jamanetworkopen.2021.47375
Development and Validation of a Treatment Benefit Index to Identify Hospitalized Patients With COVID-19 Who May Benefit From Convalescent Plasma
Abstract
Importance: Identifying which patients with COVID-19 are likely to benefit from COVID-19 convalescent plasma (CCP) treatment may have a large public health impact.
Objective: To develop an index for predicting the expected relative treatment benefit from CCP compared with treatment without CCP for patients hospitalized for COVID-19 using patients' baseline characteristics.
Design, setting, and participants: This prognostic study used data from the COMPILE study, ie, a meta-analysis of pooled individual patient data from 8 randomized clinical trials (RCTs) evaluating CCP vs control in adults hospitalized for COVID-19 who were not receiving mechanical ventilation at randomization. A combination of baseline characteristics, termed the treatment benefit index (TBI), was developed based on 2287 patients in COMPILE using a proportional odds model, with baseline characteristics selected via cross-validation. The TBI was externally validated on 4 external data sets: the Expanded Access Program (1896 participants), a study conducted under Emergency Use Authorization (210 participants), and 2 RCTs (with 80 and 309 participants).
Exposure: Receipt of CCP.
Main outcomes and measures: World Health Organization (WHO) 11-point ordinal COVID-19 clinical status scale and 2 derivatives of it (ie, WHO score of 7-10, indicating mechanical ventilation to death, and WHO score of 10, indicating death) at day 14 and day 28 after randomization. Day 14 WHO 11-point ordinal scale was used as the primary outcome to develop the TBI.
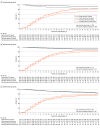
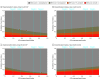
Results: A total of 2287 patients were included in the derivation cohort, with a mean (SD) age of 60.3 (15.2) years and 815 (35.6%) women. The TBI provided a continuous gradation of benefit, and, for clinical utility, it was operationalized into groups of expected large clinical benefit (B1; 629 participants in the derivation cohort [27.5%]), moderate benefit (B2; 953 [41.7%]), and potential harm or no benefit (B3; 705 [30.8%]). Patients with preexisting conditions (diabetes, cardiovascular and pulmonary diseases), with blood type A or AB, and at an early COVID-19 stage (low baseline WHO scores) were expected to benefit most, while those without preexisting conditions and at more advanced stages of COVID-19 could potentially be harmed. In the derivation cohort, odds ratios for worse outcome, where smaller odds ratios indicate larger benefit from CCP, were 0.69 (95% credible interval [CrI], 0.48-1.06) for B1, 0.82 (95% CrI, 0.61-1.11) for B2, and 1.58 (95% CrI, 1.14-2.17) for B3. Testing on 4 external datasets supported the validation of the derived TBIs.
Conclusions and relevance: The findings of this study suggest that the CCP TBI is a simple tool that can quantify the relative benefit from CCP treatment for an individual patient hospitalized with COVID-19 that can be used to guide treatment recommendations. The TBI precision medicine approach could be especially helpful in a pandemic.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

