A stochastically curtailed single-arm phase II trial design for binary outcomes
- PMID: 35077268
- PMCID: PMC7614398
- DOI: 10.1080/10543406.2021.2009498
A stochastically curtailed single-arm phase II trial design for binary outcomes
Abstract
Phase II clinical trials are a critical aspect of the drug development process. With drug development costs ever increasing, novel designs that can improve the efficiency of phase II trials are extremely valuable.Phase II clinical trials for cancer treatments often measure a binary outcome. The final trial decision is generally to continue or cease development. When this decision is based solely on the result of a hypothesis test, the result may be known with certainty before the planned end of the trial. Unfortunately, there is often no opportunity for early stopping when this occurs.Some existing designs do permit early stopping in this case, accordingly reducing the required sample size and potentially speeding up drug development. However, more improvements can be achieved by stopping early when the final trial decision is very likely, rather than certain, known as stochastic curtailment. While some authors have proposed approaches of this form, these approaches have various limitations.In this work we address these limitations by proposing new design approaches for single-arm phase II binary outcome trials that use stochastic curtailment. We use exact distributions, avoid simulation, consider a wider range of possible designs and permit early stopping for promising treatments. As a result, we are able to obtain trial designs that have considerably reduced sample sizes on average.
Keywords: Adaptive design; cancer; continuous monitoring; interim analysis; oncology.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
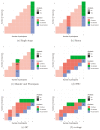

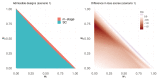
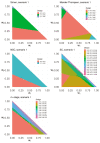


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
