Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing
- PMID: 35077667
- PMCID: PMC8988390
- DOI: 10.1016/j.stem.2021.12.011
Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing
Abstract
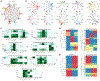
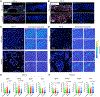
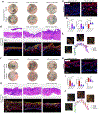
Regeneration is the holy grail of tissue repair, but skin injury typically yields fibrotic, non-functional scars. Developing pro-regenerative therapies requires rigorous understanding of the molecular progression from injury to fibrosis or regeneration. Here, we report the divergent molecular events driving skin wound cells toward scarring or regenerative fates. We profile scarring versus YAP-inhibition-induced wound regeneration at the transcriptional (single-cell RNA sequencing), protein (timsTOF proteomics), and tissue (extracellular matrix ultrastructural analysis) levels. Using cell-surface barcoding, we integrate these data to reveal fibrotic and regenerative "molecular trajectories" of healing. We show that disrupting YAP mechanotransduction yields regenerative repair by fibroblasts with activated Trps1 and Wnt signaling. Finally, via in vivo gene knockdown and overexpression in wounds, we identify Trps1 as a key regulatory gene that is necessary and partially sufficient for wound regeneration. Our findings serve as a multi-omic map of wound regeneration and could have therapeutic implications for pathologic fibroses.
Keywords: fibroblast heterogeneity; fibrosis; mechanotransduction signaling; regeneration; wound healing.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.T.L., S.M., H.E.T., and M.F.D. are inventors on patent 62/879,369 held by Stanford University that covers the use of YAP inhibition for wound healing. S.M., H.E.T., and M.T.L. are inventors on patent application PCT/US2020/043717 that covers a machine-learning algorithm for analysis of connective tissue networks in scarring and chronic fibroses. The authors declare no other competing interests.
Figures



Comment in
-
Cut out that YAPping: Mechanisms to reduce scar formation.Cell Stem Cell. 2022 Feb 3;29(2):179-181. doi: 10.1016/j.stem.2022.01.005. Cell Stem Cell. 2022. PMID: 35120615
References
-
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P, et al. (2020). Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27, 396. e6–412.e6.. 10.1016/j.stem.2020.07.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

