Insights into the specificity for the interaction of the promiscuous SARS-CoV-2 nucleocapsid protein N-terminal domain with deoxyribonucleic acids
- PMID: 35077748
- PMCID: PMC8783401
- DOI: 10.1016/j.ijbiomac.2022.01.121
Insights into the specificity for the interaction of the promiscuous SARS-CoV-2 nucleocapsid protein N-terminal domain with deoxyribonucleic acids
Abstract
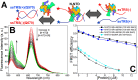
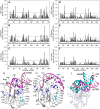
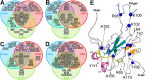
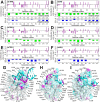
The SARS-CoV-2 nucleocapsid protein (N) is a multifunctional promiscuous nucleic acid-binding protein, which plays a major role in nucleocapsid assembly and discontinuous RNA transcription, facilitating the template switch of transcriptional regulatory sequences (TRS). Here, we dissect the structural features of the N protein N-terminal domain (N-NTD) and N-NTD plus the SR-rich motif (N-NTD-SR) upon binding to single and double-stranded TRS DNA, as well as their activities for dsTRS melting and TRS-induced liquid-liquid phase separation (LLPS). Our study gives insights on the specificity for N-NTD(-SR) interaction with TRS. We observed an approximation of the triple-thymidine (TTT) motif of the TRS to β-sheet II, giving rise to an orientation difference of ~25° between dsTRS and non-specific sequence (dsNS). It led to a local unfavorable energetic contribution that might trigger the melting activity. The thermodynamic parameters of binding of ssTRSs and dsTRS suggested that the duplex dissociation of the dsTRS in the binding cleft is entropically favorable. We showed a preference for TRS in the formation of liquid condensates when compared to NS. Moreover, our results on DNA binding may serve as a starting point for the design of inhibitors, including aptamers, against N, a possible therapeutic target essential for the virus infectivity.
Keywords: Binding specificity; DNA/RNA binding protein; SARS-CoV-2 nucleocapsid protein.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures



References
-
- F. Jiang L.Deng., L. Zhang COVID-19. J. Gen. Intern. Med. 2019;2020:1–5. doi: 10.1007/s11606-020-05762-w. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

