The essential roles of m6A RNA modification to stimulate ENO1-dependent glycolysis and tumorigenesis in lung adenocarcinoma
- PMID: 35078505
- PMCID: PMC8788079
- DOI: 10.1186/s13046-021-02200-5
The essential roles of m6A RNA modification to stimulate ENO1-dependent glycolysis and tumorigenesis in lung adenocarcinoma
Abstract
Background: Lung adenocarcinoma (LUAD) is the most common subtype of lung cancer. Patient prognosis is poor, and the existing therapeutic strategies for LUAD are far from satisfactory. Recently, targeting N6-methyladenosine (m6A) modification of RNA has been suggested as a potential strategy to impede tumor progression. However, the roles of m6A modification in LUAD tumorigenesis is unknown.
Methods: Global m6A levels and expressions of m6A writers, erasers and readers were evaluated by RNA methylation assay, dot blot, immunoblotting, immunohistochemistry and ELISA in human LUAD, mouse models and cell lines. Cell viability, 3D-spheroid generation, in vivo LUAD formation, experiments in cell- and patient-derived xenograft mice and survival analysis were conducted to explore the impact of m6A on LUAD. The RNA-protein interactions, translation, putative m6A sites and glycolysis were explored in the investigation of the mechanism underlying how m6A stimulates tumorigenesis.
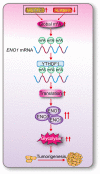
Results: The elevation of global m6A level in most human LUAD specimens resulted from the combined upregulation of m6A writer methyltransferase 3 (METTL3) and downregulation of eraser alkB homolog 5 (ALKBH5). Elevated global m6A level was associated with a poor overall survival in LUAD patients. Reducing m6A levels by knocking out METTL3 and overexpressing ALKBH5 suppressed 3D-spheroid generation in LUAD cells and intra-pulmonary tumor formation in mice. Mechanistically, m6A-dependent stimulation of glycolysis and tumorigenesis occurred via enolase 1 (ENO1). ENO1 mRNA was m6A methylated at 359 A, which facilitated it's binding with the m6A reader YTH N6-methyladenosine RNA binding protein 1 (YTHDF1) and resulted in enhanced translation of ENO1. ENO1 positively correlated with METTL3 and global m6A levels, and negatively correlated with ALKBH5 in human LUAD. In addition, m6A-dependent elevation of ENO1 was associated with LUAD progression. In preclinical models, tumors with a higher global m6A level showed a more sensitive response to the inhibition of pan-methylation, glycolysis and ENO activity in LUAD.
Conclusions: The m6A-dependent stimulation of glycolysis and tumorigenesis in LUAD is at least partially orchestrated by the upregulation of METTL3, downregulation of ALKBH5, and stimulation of YTHDF1-mediated ENO1 translation. Blocking this mechanism may represent a potential treatment strategy for m6A-dependent LUAD.
Keywords: ALKBH5; METTL3; RNA-protein interaction; YTHDF1; lung cancer; translation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures







References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
MeSH terms
Substances
Grants and funding
- 81871907, 81822029, 82173015, 82102792, 81872288, 81902315, 81902869, 81774291/National Natural Science Foundation of China
- 20191834/Shanghai Municipal Education Commission-Gaofeng Clinical Medicine
- Project of Clinical Research Supporting System, Clinical Medicine First-class Discipline/Project of Clinical Research Supporting System, Clinical Medicine First-class Discipline
- Talent training plan of shanghai chest hospital in 2020/Talent training plan of shanghai chest hospital in 2020
- 19YF1444800/Shanghai Sailing Program
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

