ATR Inhibitor AZD6738 (Ceralasertib) Exerts Antitumor Activity as a Monotherapy and in Combination with Chemotherapy and the PARP Inhibitor Olaparib
- PMID: 35078817
- PMCID: PMC9359726
- DOI: 10.1158/0008-5472.CAN-21-2997
ATR Inhibitor AZD6738 (Ceralasertib) Exerts Antitumor Activity as a Monotherapy and in Combination with Chemotherapy and the PARP Inhibitor Olaparib
Abstract
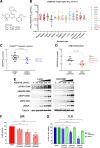
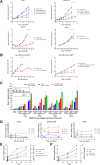
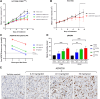
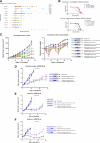
AZD6738 (ceralasertib) is a potent and selective orally bioavailable inhibitor of ataxia telangiectasia and Rad3-related (ATR) kinase. ATR is activated in response to stalled DNA replication forks to promote G2-M cell-cycle checkpoints and fork restart. Here, we found AZD6738 modulated CHK1 phosphorylation and induced ATM-dependent signaling (pRAD50) and the DNA damage marker γH2AX. AZD6738 inhibited break-induced replication and homologous recombination repair. In vitro sensitivity to AZD6738 was elevated in, but not exclusive to, cells with defects in the ATM pathway or that harbor putative drivers of replication stress such as CCNE1 amplification. This translated to in vivo antitumor activity, with tumor control requiring continuous dosing and free plasma exposures, which correlated with induction of pCHK1, pRAD50, and γH2AX. AZD6738 showed combinatorial efficacy with agents associated with replication fork stalling and collapse such as carboplatin and irinotecan and the PARP inhibitor olaparib. These combinations required optimization of dose and schedules in vivo and showed superior antitumor activity at lower doses compared with that required for monotherapy. Tumor regressions required at least 2 days of daily dosing of AZD6738 concurrent with carboplatin, while twice daily dosing was required following irinotecan. In a BRCA2-mutant patient-derived triple-negative breast cancer (TNBC) xenograft model, complete tumor regression was achieved with 3 to5 days of daily AZD6738 per week concurrent with olaparib. Increasing olaparib dosage or AZD6738 dosing to twice daily allowed complete tumor regression even in a BRCA wild-type TNBC xenograft model. These preclinical data provide rationale for clinical evaluation of AZD6738 as a monotherapy or combinatorial agent.
Significance: This detailed preclinical investigation, including pharmacokinetics/pharmacodynamics and dose-schedule optimizations, of AZD6738/ceralasertib alone and in combination with chemotherapy or PARP inhibitors can inform ongoing clinical efforts to treat cancer with ATR inhibitors.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Forment JV, O'Connor MJ. Targeting the replication stress response in cancer. Pharmacol Ther 2018;188:155–67. - PubMed
-
- Foote KM, Lau A, Nissink JW. Drugging ATR: progress in the development of specific inhibitors for the treatment of cancer. Future Med Chem 2015;7:873–91. - PubMed
-
- Sundar R, Brown J, Ingles Russo A, Yap TA. Targeting ATR in cancer medicine. Curr Probl Cancer 2017;41:302–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

