Communication, Coordination, and Security for People with Multiple Sclerosis (COCOS-MS): a randomised phase II clinical trial protocol
- PMID: 35078833
- PMCID: PMC8796263
- DOI: 10.1136/bmjopen-2021-049300
Communication, Coordination, and Security for People with Multiple Sclerosis (COCOS-MS): a randomised phase II clinical trial protocol
Abstract
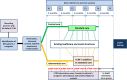
Introduction: Patients with multiple sclerosis (MS) have complex needs that range from organising one's everyday life to measures of disease-specific therapy monitoring to palliative care. Patients with MS are likely to depend on multiple healthcare providers and various authorities, which are often difficult to coordinate. Thus, they will probably benefit from comprehensive cross-sectoral coordination of services provided by care and case management (CCM). Though studies have shown that case management improves quality of life (QoL), functional status and reduces service use, such benefits have not yet been investigated in severely affected patients with MS. In this explorative phase ll clinical trial, we evaluated a CCM with long-term, cross-sectoral and outreaching services and, in addition, considered the unit of care (patients and caregivers).
Methods and analysis: Eighty patients with MS and their caregivers will be randomly assigned to either the control (standard care) or the intervention group (standard care plus CCM (for 12 months)). Regular data assessments will be done at baseline and then at 3-month intervals. As primary outcome, we will evaluate patients' QoL. Secondary outcomes are patients' treatment-related risk perception, palliative care needs, anxiety/depression, use of healthcare services, caregivers' burden and QoL, meeting patients' and caregivers' needs, and evaluating the CCM intervention. We will also evaluate CCM through individual interviews and focus groups. The sample size calculation is based on a standardised effect of 0.5, and one baseline and four follow-up assessments (with correlation 0.5). Linear mixed models for repeated measures will be applied to analyse changes in quantitative outcomes over time. Multiple imputation approaches are taken to assess the robustness of the results. The explorative approach (phase ll clinical trial) with embedded qualitative research will allow for the development of a final design for a confirmative phase lll trial.
Ethics and dissemination: The trial will be conducted under the Declaration of Helsinki and has been approved by the Ethics Commission of Cologne University's Faculty of Medicine. Trial results will be published in an open-access scientific journal and presented at conferences.
Trial registration number: German Register for Clinical Studies (DRKS) (DRKS00022771).
Keywords: adult palliative care; multiple sclerosis; quality in health care.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
"So at least now I know how to deal with things myself, what I can do if it gets really bad again"-experiences with a long-term cross-sectoral advocacy care and case management for severe multiple sclerosis: a qualitative study.BMC Health Serv Res. 2024 Apr 10;24(1):453. doi: 10.1186/s12913-024-10851-1. BMC Health Serv Res. 2024. PMID: 38600493 Free PMC article.
-
Effect of early palliative care for patients with glioblastoma (EPCOG): a randomised phase III clinical trial protocol.BMJ Open. 2020 Jan 7;10(1):e034378. doi: 10.1136/bmjopen-2019-034378. BMJ Open. 2020. PMID: 31915175 Free PMC article.
-
Development of a Long-Term Cross-Sectoral Case and Care Management Manual for Patients With Severe Multiple Sclerosis and Their Caregivers.Prof Case Manag. 2023 Jul-Aug 01;28(4):183-193. doi: 10.1097/NCM.0000000000000608. Epub 2022 Dec 14. Prof Case Manag. 2023. PMID: 36518082 Clinical Trial.
-
Palliative care interventions for people with multiple sclerosis.Cochrane Database Syst Rev. 2019 Oct 22;10(10):CD012936. doi: 10.1002/14651858.CD012936.pub2. Cochrane Database Syst Rev. 2019. PMID: 31637711 Free PMC article. Review.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
Cited by
-
The economic burden of individuals living with generalized myasthenia gravis and facing social determinants of health challenges.Front Public Health. 2023 Sep 12;11:1247931. doi: 10.3389/fpubh.2023.1247931. eCollection 2023. Front Public Health. 2023. PMID: 37766748 Free PMC article.
-
Current Practices, Challenges, and Future Directions in Multiple Sclerosis Management in Sub-Saharan Africa.Int J MS Care. 2025 Apr 15;27(Theme):T13-T16. doi: 10.7224/1537-2073.2024-080. eCollection 2024 Dec. Int J MS Care. 2025. PMID: 40241912 Free PMC article.
-
Persons with multiple sclerosis older than 55 years: an analysis from the German MS registry.J Neurol. 2024 Jun;271(6):3409-3416. doi: 10.1007/s00415-024-12286-4. Epub 2024 Mar 22. J Neurol. 2024. PMID: 38517521 Free PMC article.
-
"So at least now I know how to deal with things myself, what I can do if it gets really bad again"-experiences with a long-term cross-sectoral advocacy care and case management for severe multiple sclerosis: a qualitative study.BMC Health Serv Res. 2024 Apr 10;24(1):453. doi: 10.1186/s12913-024-10851-1. BMC Health Serv Res. 2024. PMID: 38600493 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials