Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies
- PMID: 35078922
- PMCID: PMC8796265
- DOI: 10.1136/jitc-2021-003024
Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies
Erratum in
-
Correction: Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies.J Immunother Cancer. 2022 Dec;10(12):e003024corr1. doi: 10.1136/jitc-2021-003024corr1. J Immunother Cancer. 2022. PMID: 36543380 Free PMC article. No abstract available.
Abstract
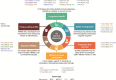
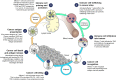
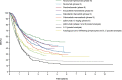
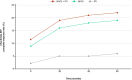
IntroductionImmuno-oncology therapies, including immune checkpoint inhibitors (ICIs), have transformed cancer care and have brought into question whether classic oncology efficacy assessments adequately describe the distinctive responses observed with these agents. With more ICI-based therapies being approved across multiple tumor types, it is essential to define unique clinical hallmarks of these agents and their associated assessments to better reflect the therapeutic impact for both patients and physicians. Long-term survival and objective responses, such as depth and durability of responses, treatment-free survival, efficacy in brain metastases, improved health-related quality of life, and unique safety profiles, are among the hallmarks that have emerged for ICI therapies. An established clinical hallmark is a sustained long-term survival, as evidenced by a delayed separation of Kaplan-Meier survival curves, and a plateau at ~3 years. Combination ICI therapies provide the opportunity to raise this plateau, thereby affording durable survival benefits to more patients. Deepening of responses over time is a unique clinical ICI hallmark, with patients responding long term and with more durable complete responses. Depth of response has demonstrated prognostic value for long-term survival in some cancers, and several ICI studies have shown sustained responses even after discontinuing ICI therapy, offering the potential for treatment-free intervals. Although clinical evidence supporting efficacy in brain metastases is limited, favorable ICI intracranial responses have been seen that are largely concordant with extracranial responses. While patient outcomes can be significantly improved with ICIs, they are associated with unique immune-mediated adverse reactions (IMARs), including delayed ICI toxicities, and may require multidisciplinary management for optimal care. Interestingly, patients discontinuing ICIs for IMARs may maintain responses similar to patients who did not discontinue for an IMAR, whether they restarted ICI therapy or not.ConclusionHerein, we comprehensively review and refine the clinical hallmarks uniquely associated with ICI therapies, which not only will rejuvenate our assessment of ICI therapeutic outcomes but also will lead to a greater appreciation of the effectiveness of ICI therapies.
Keywords: CTLA-4 antigen; combination; drug therapy; immunotherapy; programmed cell death 1 receptor; review.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SP received education grants and honoraria from and provided consultation, attended advisory boards, and/or provided lectures for AbbVie, Amgen, AstraZeneca, Bayer, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffmann-La Roche, Foundation Medicine, Illumina, Incyte, Janssen, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, Pharma Mar, Pfizer, Regeneron, Sanofi, Seattle Genetics, and Takeda. SP also talked at an organized public event for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Illumina, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, and Takeda, and received grants and research supports as a (sub)investigator in trials (institutional financial support for clinical trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, F. Hoffmann-La Roche, Illumina, Merck Sharp and Dohme, Merck Serono, Novartis, and Pfizer. A-KL received grants or research support, institutionally, from Bristol Myers Squibb, BioCanRx, Novartis, Roche, Ipsen, and EMD Serono, and was in an advisory or consulting role for AbbVie, Astellas, Bristol Myers Squibb, Eisai, Ipsen, Janssen, Merck, Novartis, Pfizer, Roche, and TerSera. OM received grants and personal fees from Bristol Myers Squibb and Merck Sharp and Dohme, Pierre-Fabre and Amgen, and personal fees from Roche, Novartis, GSK, and Merck, outside the submitted work. CR was a consultant for Amgen, Bristol Myers Squibb, Novartis, Roche, Merck Sharp and Dohme, Pierre Fabre, and AstraZeneca. PS has received consulting fees or stock ownership or attended advisory boards for Achelois, Adaptive Biotechnologies, Affini-T, Apricity, BioAtla, BioNTech, Codiak, Constellation, Dragonfly, Earli, Glympse, Hummingbird, ImaginAb, Infinity Pharma, Jounce, JSL Health, Lava Therapeutics, Lytix, Marker, Oncolytics, PBM Capital, Phenomics, Polaris, Sporos, Time BioVentures, and Venn Biosciences.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
