Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer
- PMID: 35078923
- PMCID: PMC8796267
- DOI: 10.1136/jitc-2021-003747
Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer
Abstract
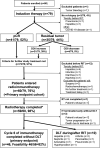
Purpose: The first aim of the trial is to study feasibility of combined programmed death protein ligand 1/cytotoxic T-lymphocyte-associated protein 4 inhibition concomitant to radiotherapy. In addition, efficacy of the entire treatment scheme consisting of induction chemoimmunotherapy followed by chemotherapy-free radioimmunotherapy (RIT) after intratumoral CD8 +immune cell-based patient selection will be analyzed.
Methods: Patients with stage III-IVB head and neck squamous cell carcinoma were eligible for this multicenter phase II trial. Treatment consisted of a single cycle of cisplatin 30 mg/m² days 1-3, docetaxel 75 mg/m² day 1, durvalumab 1500 mg fix dose day 5 and tremelimumab 75 mg fix dose day 5. Patients with increased intratumoral CD8 +immune cell density or pathological complete response (pCR) in the rebiopsy entered RIT up to a total dose of 70 Gy. Patients received further three cycles of durvalumab/tremelimumab followed by eight cycles of durvalumab mono (every 4 weeks). The intended treatment for patients not meeting these criteria was standard radiochemotherapy outside the trial. Primary endpoint was a feasibility rate of patients entering RIT to receive treatment until at least cycle 6 of immunotherapy of ≥80%.
Results: Between September 2018 and May 2020, 80 patients were enrolled (one excluded). Out of these, 23 patients had human papilloma virus (HPV)-positive oropharyngeal cancer. Median follow-up was 17.2 months. After induction chemoimmunotherapy 41 patients had pCR and 31 had increased intratumoral CD8 +immune cells. Of 60 patients entering RIT (primary endpoint cohort), 10 experienced imiting toxic (mainly hepatitis) and four discontinued for other reasons, resulting in a feasibility rate of 82%. The RIT cohort (n=60) had a progression-free survival (PFS) rate at one and 2 years of 78% and 72%, respectively, and an overall survival rate at one and 2 years of 90% and 84%, respectively. Patients with HPV-positive oropharyngeal cancers had greater benefit from RIT with a 2-year PFS rate of 94% compared with 64% for HPV-negative oropharyngeal cancers and other locations. In the entire study cohort (n=79) the 2-year PFS rate was 68% (91% for HPV-positive oropharynx vs 59% for others). Toxicity grade 3-4 mainly consisted of dysphagia (53%), leukopenia (52%) and infections (32%).
Conclusions: The trial met the primary endpoint feasibility of RIT. Induction chemo-immunotherapy followed by chemotherapy-free RIT after intratumoral CD8 +immune cell-based patient selection has promising PFS.
Trial registration number: The trial was registered with ClinicalTrials.gov (identifier: NCT03426657). The trial was conducted as investigator-sponsored trial (IST).
Keywords: radioimmunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MH conflict of interest with Merck Serono (advisory role, speakers’ bureau, honoraria, travel expenses, research funding); MSD (advisory role, speakers’ bureau, honoraria, travel expenses, research funding); AstraZeneca (research funding); Novartis (research funding); BMS (advisory role, honoraria, speakers’ bureau); Teva (travel expenses). ME conflict of interest with Diaceutics (employment, honoraria, advisory role, speakers’ bureau, travel expenses); Cepheid (research funding, advisory role); AstraZeneca (honoraria, advisory role, speakers’ bureau, travel expenses); Roche (honoraria, travel expenses); MSD (honoraria, speakers’ bureau); GenomicHealth (honoraria, advisory role, speakers bureau, travel expenses); Astellas (honoraria, speakers’ bureau); Janssen-Cilag (honoraria, advisory role, research funding, travel expenses); Stratifyer (research funding, patents). SR conflict of interest with AstraZeneca (research funding); MSD (research funding). GK conflict of interest with BMS (advisory role); Lilly (advisory role); Roche (advisory role). SL conflict of interest with AstraZeneca (honoraria, advisory role); BMS (honoraria, advisory role, speakers’ bureau); MSD (honoraria, advisory role); Merck Serono (honoraria, speakers’ bureau); ISA-Pharmaceuticals (research funding). MGH conflict of interest with Roche (stock); Varian (stock); Sanofi (stock); AstraZeneca (honoraria); BMS (honoraria, advisory role); MSD (honoraria, advisory role); Merck Serono (honoraria); Celgene (honoraria). AHi conflict of interest with Roche (honoraria). SS conflict of interest with Strycker (stock); Varian (stock); Abbot (stock); Crispr Techn. (stock); Pfitzer (stock); Merck Serono (stock); Symrise (stock); Ortho (honoraria, advisory role, speakers’ bureau, research funding, travel expenses); PharmaMar (speakers’ bureau, travel expenses); Haema (speakers’ bureau). AHa. conflict of interest with BMS (honoraria, advisory role); MSD (honoraria, advisory role); Roche (honoraria, advisory role, research funding); AstraZeneca (honoraria, advisory role, research funding); Boehringer Ingelheim (honoraria); Abbvie (honoraria); Cepheid (advisory role, research funding); Quiagen (advisory role); Janssen-Cilag (honoraria, advisory role, research funding); Ipsen (honoraria, advisory role); NanoString Technologies (advisory role, research funding, expert testimony); Illumina (advisory role); 3DHistech (advisory role); Diaceutics (advisory role); BioNTech (research funding). WB conflict of interest with BMS (advisory role); MSD (advisory role); Merck Serono (advisory role); Pfitzer (advisory role); AstraZeneca (advisory role). UG conflict of interest with AstraZeneca (advisory role, research funding); BMS (advisory role); MSD (research funding); Sennewald Medizintechnik (advisory role, travel expenses). RF conflict of interest with MSD (honoraria, advisory role, research funding, travel expenses); Fresenius (honoraria); BrainLab (honoraria); AstraZeneca (honoraria, advisory role, research funding, travel expenses); Merck Serono (advisory role, research funding, travel expenses); Novocure (advisory role, speakers’ bureau, research funding); Sennewald (speakers’ bureau, travel expenses). The other authors declare no conflicts of interest.
Figures


References
-
- Burtness B, Harrington KJ, Greil R, et al. . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. 10.1016/S0140-6736(19)32591-7 - DOI - PubMed
-
- Lee NY, Ferris RL, Psyrri A, et al. . Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 2021;22:450–62. 10.1016/S1470-2045(20)30737-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
