Viral vector-based gene therapies in the clinic
- PMID: 35079633
- PMCID: PMC8780015
- DOI: 10.1002/btm2.10258
Viral vector-based gene therapies in the clinic
Abstract
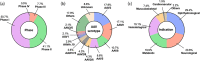
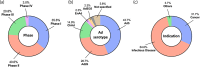
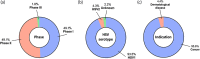
Gene therapies are currently one of the most investigated therapeutic modalities in both the preclinical and clinical settings and have shown promise in treating a diverse spectrum of diseases. Gene therapies aim at introducing a gene material in target cells and represent a promising approach to cure diseases that were thought to be incurable by conventional modalities. In many cases, a gene therapy requires a vector to deliver gene therapeutics into target cells; viral vectors are among the most widely studied vectors owing to their distinguished advantages such as outstanding transduction efficiency. With decades of development, viral vector-based gene therapies have achieved promising clinical outcomes with many products approved for treating a range of diseases including cancer, infectious diseases and monogenic diseases. In addition, a number of active clinical trials are underway to further expand their therapeutic potential. In this review, we highlight the diversity of viral vectors, review approved products, and discuss the current clinical landscape of in vivo viral vector-based gene therapies. We have reviewed 13 approved products and their clinical applications. We have also analyzed more than 200 active trials based on various viral vectors and discussed their respective therapeutic applications. Moreover, we provide a critical analysis of the major translational challenges for in vivo viral vector-based gene therapies and discuss possible strategies to address the same.
Keywords: adenovirus; adeno‐associated virus; clinical translation; clinical trials; gene; gene therapy; gene transfer; herpes simplex virus; viral vector.
© 2021 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Figures


References
-
- Verma IM, Somia N. Gene therapy–promises, problems and prospects. Nature. 1997;389(6648):239‐242. - PubMed
-
- Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372):eaan4672. - PubMed
-
- Cavazzana‐Calvo M, Thrasher A, Mavilio F. The future of gene therapy. Nature. 2004;427(6977):779‐781. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

