Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation
- PMID: 35079788
- PMCID: PMC9398450
- DOI: 10.1093/humrep/deac008
Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation
Abstract
Study question: How does the human embryo breach the endometrial epithelium at implantation?
Summary answer: Embryo attachment to the endometrial epithelium promotes the formation of multinuclear syncytiotrophoblast from trophectoderm, which goes on to breach the epithelial layer.
What is known already: A significant proportion of natural conceptions and assisted reproduction treatments fail due to unsuccessful implantation. The trophectoderm lineage of the embryo attaches to the endometrial epithelium before breaching this barrier to implant into the endometrium. Trophectoderm-derived syncytiotrophoblast has been observed in recent in vitro cultures of peri-implantation embryos, and historical histology has shown invasive syncytiotrophoblast in embryos that have invaded beyond the epithelium, but the cell type mediating invasion of the epithelial layer at implantation is unknown.
Study design, size, duration: Fresh and frozen human blastocyst-stage embryos (n = 46) or human trophoblast stem cell (TSC) spheroids were co-cultured with confluent monolayers of the Ishikawa endometrial epithelial cell line to model the epithelial phase of implantation in vitro. Systems biology approaches with published transcriptomic datasets were used to model the epithelial phase of implantation in silico.
Participants/materials, setting, methods: Human embryos surplus to treatment requirements were consented for research. Day 6 blastocysts were co-cultured with Ishikawa cell layers until Day 8, and human TSC spheroids modelling blastocyst trophectoderm were co-cultured with Ishikawa cell layers for 48 h. Embryo and TSC morphology was assessed by immunofluorescence microscopy, and TSC differentiation by real-time quantitative PCR (RT-qPCR) and ELISA. Single-cell human blastocyst transcriptomes, and bulk transcriptomes of TSC and primary human endometrial epithelium were used to model the trophectoderm-epithelium interaction in silico. Hypernetworks, pathway analysis, random forest machine learning and RNA velocity were employed to identify gene networks associated with implantation.
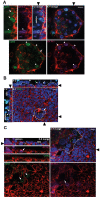
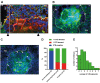
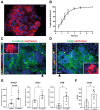
Main results and the role of chance: The majority of embryos co-cultured with Ishikawa cell layers from Day 6 to 8 breached the epithelial layer (37/46), and syncytiotrophoblast was seen in all of these. Syncytiotrophoblast was observed at the embryo-epithelium interface before breaching, and syncytiotrophoblast mediated all pioneering breaching events observed (7/7 events). Multiple independent syncytiotrophoblast regions were seen in 26/46 embryos, suggesting derivation from different regions of trophectoderm. Human TSC spheroids co-cultured with Ishikawa layers also exhibited syncytiotrophoblast formation upon invasion into the epithelium. RT-qPCR comparison of TSC spheroids in isolated culture and co-culture demonstrated epithelium-induced upregulation of syncytiotrophoblast genes CGB (P = 0.03) and SDC1 (P = 0.008), and ELISA revealed the induction of hCGβ secretion (P = 0.03). Secretory-phase primary endometrial epithelium surface transcriptomes were used to identify trophectoderm surface binding partners to model the embryo-epithelium interface. Hypernetwork analysis established a group of 25 epithelium-interacting trophectoderm genes that were highly connected to the rest of the trophectoderm transcriptome, and epithelium-coupled gene networks in cells of the polar region of the trophectoderm exhibited greater connectivity (P < 0.001) and more organized connections (P < 0.0001) than those in the mural region. Pathway analysis revealed a striking similarity with syncytiotrophoblast differentiation, as 4/6 most highly activated pathways upon TSC-syncytiotrophoblast differentiation (false discovery rate (FDR < 0.026)) were represented in the most enriched pathways of epithelium-coupled gene networks in both polar and mural trophectoderm (FDR < 0.001). Random forest machine learning also showed that 80% of the endometrial epithelium-interacting trophectoderm genes identified in the hypernetwork could be quantified as classifiers of TSC-syncytiotrophoblast differentiation. This multi-model approach suggests that invasive syncytiotrophoblast formation from both polar and mural trophectoderm is promoted by attachment to the endometrial epithelium to enable embryonic invasion.
Large scale data: No omics datasets were generated in this study, and those used from previously published studies are cited.
Limitations, reasons for caution: In vitro and in silico models may not recapitulate the dynamic embryo-endometrial interactions that occur in vivo. The influence of other cellular compartments in the endometrium, including decidual stromal cells and leukocytes, was not represented in these models.
Wider implications of the findings: Understanding the mechanism of human embryo breaching of the epithelium and the gene networks involved is crucial to improve implantation success rates after assisted reproduction. Moreover, early trophoblast lineages arising at the epithelial phase of implantation form the blueprint for the placenta and thus underpin foetal growth trajectories, pregnancy health and offspring health.
Study funding/competing interest(s): This work was funded by grants from Wellbeing of Women, Diabetes UK, the NIHR Local Comprehensive Research Network and Manchester Clinical Research Facility, and the Department of Health Scientist Practitioner Training Scheme. None of the authors has any conflict of interest to declare.
Keywords: cell culture; embryo development; gene expression; implantation; trophoblasts.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Aberkane A, Essahib W, Spits C, De Paepe C, Sermon K, Adriaenssens T, Mackens S, Tournaye H, Brosens JJ, Van de Velde H.. Expression of adhesion and extracellular matrix genes in human blastocysts upon attachment in a 2D co-culture system. Mol Hum Reprod 2018;24:375–387. - PubMed
-
- Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod 2005;20:3369–3375. - PubMed
-
- Altmae S, Reimand J, Hovatta O, Zhang P, Kere J, Laisk T, Saare M, Peters M, Vilo J, Stavreus-Evers A. et al. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol 2012;26:203–217. - PMC - PubMed
-
- Aplin JD. Developmental cell biology of human villous trophoblast: current research problems. Int J Dev Biol 2010;54:323–329. - PubMed
-
- Aplin JD, Jones CJ, Harris LK.. Adhesion molecules in human trophoblast—a review. I. Villous trophoblast. Placenta 2009;30:293–298. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

