Efficacy and Safety of Switching to Dolutegravir/Lamivudine Versus Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Results Through Week 144 From the Phase 3, Noninferiority TANGO Randomized Trial
- PMID: 35079789
- PMCID: PMC9639798
- DOI: 10.1093/cid/ciac036
Efficacy and Safety of Switching to Dolutegravir/Lamivudine Versus Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Results Through Week 144 From the Phase 3, Noninferiority TANGO Randomized Trial
Abstract
Background: Switching to dolutegravir/lamivudine (DTG/3TC) was noninferior to continuing tenofovir alafenamide (TAF)-based regimens for maintaining virologic suppression at week 48 of the TANGO study. Here we present week 144 outcomes (efficacy, safety, weight, and biomarkers).
Methods: TANGO is a randomized (1:1, stratified by baseline third agent class), open-label, noninferiority phase 3 study. Virologically suppressed (>6 months) adults with human immunodeficiency virus type 1 (HIV-1) switched to once-daily DTG/3TC or continued TAF-based regimens.
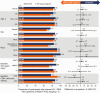
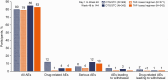
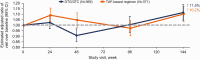
Results: A total of 741 participants received study treatment (DTG/3TC, n = 369; TAF-based regimen, n = 372). At week 144, the proportion of participants with an HIV-1 RNA level ≥50 copies/mL (primary end point, Snapshot; intention-to-treat-exposed population) after switching to DTG/3TC was 0.3% (1 of 369) versus 1.3% (5 of 372) for those continuing TAF-based regimens, demonstrating noninferiority (adjusted treatment difference, -1.1 [95% confidence interval, -2.4 to .2), with DTG/3TC favored in the per-protocol analysis (adjusted treatment difference, -1.1 [-2.3 to -.0]; P = .04). Few participants met confirmed virologic withdrawal criteria (none in the DTG/3TC and 3 in the TAF-based regimen group), with no resistance observed. Drug-related adverse events were more frequent with DTG/3TC (15%; leading to discontinuation in 4%) than TAF-based regimens (5%; leading to discontinuation in 1%) through week 144, but rates were comparable after week 48 (4%; leading to discontinuation in 1% in both groups). Changes from baseline in lipid values generally favored DTG/3TC; no clinical impact on renal function and comparable changes in inflammatory and bone biomarkers across groups were observed.
Conclusions: Switching to DTG/3TC demonstrated noninferior and durable efficacy compared with continuing TAF-based regimens in treatment-experienced adults with HIV-1, with good safety and tolerability, and no resistance through 144 weeks.
Trial registration: ClinicalTrials.gov NCT03446573.
Keywords: 2-drug regimen; dolutegravir/lamivudine; durable; integrase strand transfer inhibitor; treatment-experienced.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. O. O. has received personal fees from ViiV Healthcare, Gilead, and Merck; has received financial support for meeting attendance/travel from GlaxoSmithKline and Merck; and owns stock in Gilead, Pfizer, and GlaxoSmithKline. S. D. W. has received financial support or grants paid to his institution from ViiV Healthcare, Gilead, Merck Sharpe & Dohme, and Janssen and has participated in data safety monitoring or advisory boards for ViiV Healthcare and Curevac. F. B. has participated in advisory boards for Gilead and is the president of AusPATH. J. P. has received grants, personal fees, and nonfinancial support from ViiV Healthcare, Gilead, and Janssen-Cilag. C. W. has received personal fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharpe & Dohme, Roche, Theratechnologies, and ViiV Healthcare for speaking at educational events or participating in advisory boards. M. A. -K., P. L., K. A. P., R. W., B. W., M. A., J. v. W., and K. Y. S. are employees of ViiV Healthcare and own stock in GlaxoSmithKline. J. W. and N. G. are employees of and own stock in GlaxoSmithKline. F. A. and J. -P. R. report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Cahn P, Sierra Madero J, Arribas JR, et al. . Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

