Trypsin enhances SARS-CoV-2 infection by facilitating viral entry
- PMID: 35079901
- PMCID: PMC8789370
- DOI: 10.1007/s00705-021-05343-0
Trypsin enhances SARS-CoV-2 infection by facilitating viral entry
Abstract
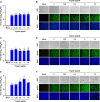
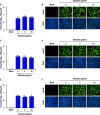
Coronaviruses infect cells by cytoplasmic or endosomal membrane fusion, driven by the spike (S) protein, which must be primed by proteolytic cleavage at the S1/S2 furin cleavage site (FCS) and the S2' site by cellular proteases. Exogenous trypsin as a medium additive facilitates isolation and propagation of several coronaviruses in vitro. Here, we show that trypsin enhances severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in cultured cells and that SARS-CoV-2 enters cells via either a non-endosomal or an endosomal fusion pathway, depending on the presence of trypsin. Interestingly, trypsin enabled viral entry at the cell surface and led to more efficient infection than trypsin-independent endosomal entry, suggesting that trypsin production in the target organs may trigger a high level of replication of SARS-CoV-2 and cause severe tissue injury. Extensive syncytium formation and enhanced growth kinetics were observed only in the presence of exogenous trypsin when cell-adapted SARS-CoV-2 strains were tested. During 50 serial passages without the addition of trypsin, a specific R685S mutation occurred in the S1/S2 FCS (681PRRAR685) that was completely conserved but accompanied by several mutations in the S2 fusion subunit in the presence of trypsin. These findings demonstrate that the S1/S2 FCS is essential for proteolytic priming of the S protein and fusion activity for SARS-CoV-2 entry but not for viral replication. Our data can potentially contribute to the improvement of SARS-CoV-2 production for the development of vaccines or antivirals and motivate further investigations into the explicit functions of cell-adaptation-related genetic drift in SARS-CoV-2 pathogenesis.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
-
- Gorbalenya AE, Baker SC, Baric RS, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. - DOI - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

