Combining information to estimate adherence in studies of pre-exposure prophylaxis for HIV prevention: Application to HPTN 067
- PMID: 35080038
- PMCID: PMC8881405
- DOI: 10.1002/sim.9321
Combining information to estimate adherence in studies of pre-exposure prophylaxis for HIV prevention: Application to HPTN 067
Abstract
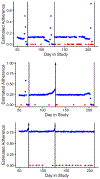
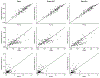
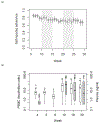
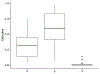
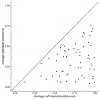
In trials of oral HIV pre-exposure prophylaxis (PrEP), multiple approaches have been used to measure adherence, including self-report, pill counts, electronic dose monitoring devices, and biological measures such as drug levels in plasma, peripheral blood mononuclear cells, hair, and/or dried blood spots. No one of these measures is ideal and each has strengths and weaknesses. However, accurate estimates of adherence to oral PrEP are important as drug efficacy is closely tied to adherence, and secondary analyses of trial data within identified adherent/non-adherent subgroups may yield important insights into real-world drug effectiveness. We develop a statistical approach to combining multiple measures of adherence and show in simulated data that the proposed method provides a more accurate measure of true adherence than self-report. We then apply the method to estimate adherence in the ADAPT study (HPTN 067) in South African women.
Trial registration: ClinicalTrials.gov NCT01327651.
Keywords: HIV; adherence; latent variable; pharmacokinetic model; pre-exposure prophylaxis.
© 2022 John Wiley & Sons Ltd.
Figures
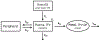
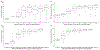
References
-
- Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med.. 2015;373:2237–2246. - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med.. 2012;367:423–434. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
