Adenovirus-α-Defensin Complexes Induce NLRP3-Associated Maturation of Human Phagocytes via Toll-Like Receptor 4 Engagement
- PMID: 35080426
- PMCID: PMC8941863
- DOI: 10.1128/jvi.01850-21
Adenovirus-α-Defensin Complexes Induce NLRP3-Associated Maturation of Human Phagocytes via Toll-Like Receptor 4 Engagement
Abstract
Intramuscular delivery of human adenovirus (HAdV)-based vaccines leads to rapid recruitment of neutrophils, which then release antimicrobial peptides/proteins (AMPs). How these AMPs influence vaccine efficacy over the subsequent 24 h is poorly understood. In this study, we asked if human neutrophil protein 1 (HNP-1), an α-defensin that influences direct and indirect innate immune responses to a range of pathogens, impacts the response of human phagocytes to three HAdV species/types (HAdV-C5, -D26, -B35). We show that HNP-1 binds to the capsids and redirects HAdV-C5, -D26, and -B35 to Toll-like receptor 4 (TLR4), which leads to internalization, an NLRP3-mediated inflammasome response, and interleukin 1 beta (IL-1β) release. Surprisingly, IL-1β release was not associated with notable disruption of plasma membrane integrity. These data further our understanding of HAdV vaccine immunogenicity and may provide pathways to extend the efficacy. IMPORTANCE This study examines the interactions between danger-associated molecular patterns and human adenoviruses, and their impact on vaccines. HAdVs and HNP-1 can interact, and these interactions will modify the response of antigen-presenting cells, which will influence vaccine efficacy.
Keywords: Toll-like receptors; adenoviruses; antimicrobial peptides; innate immunity; phagocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
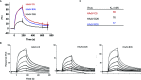
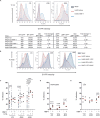





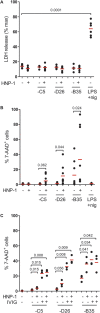
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

