Human B Cell Responses to Dominant and Subdominant Antigens Induced by a Meningococcal Outer Membrane Vesicle Vaccine in a Phase I Trial
- PMID: 35080470
- PMCID: PMC8791392
- DOI: 10.1128/msphere.00674-21
Human B Cell Responses to Dominant and Subdominant Antigens Induced by a Meningococcal Outer Membrane Vesicle Vaccine in a Phase I Trial
Abstract
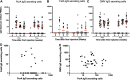
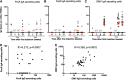
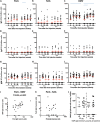
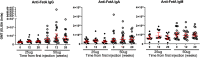
Neisseria meningitidis outer membrane vesicle (OMV) vaccines are safe and provide strain-specific protection against invasive meningococcal disease (IMD) primarily by inducing serum bactericidal antibodies against the outer membrane proteins (OMP). To design broader coverage vaccines, knowledge of the immunogenicity of all the antigens contained in OMVs is needed. In a Phase I clinical trial, an investigational meningococcal OMV vaccine, MenPF1, made from a meningococcus genetically modified to constitutively express the iron-regulated FetA induced bactericidal responses to both the PorA and the FetA antigen present in the OMP. Using peripheral blood mononuclear cells collected from this trial, we analyzed the kinetics of and relationships between IgG, IgA, and IgM B cell responses against recombinant PorA and FetA, including (i) antibody-secreting cells, (ii) memory B cells, and (iii) functional antibody responses (opsonophagocytic and bactericidal activities). Following MenPF1vaccination, PorA-specific IgG secreting cell responses were detected in up to 77% of participants and FetA-specific responses in up to 36%. Memory B cell responses to the vaccine were low or absent and mainly detected in participants who had evidence of preexisting immunity (P = 0.0069). Similarly, FetA-specific antibody titers and bactericidal activity increased in participants with preexisting immunity and is consistent with the idea that immune responses are elicited to minor antigens during asymptomatic Neisseria carriage, which can be boosted by OMV vaccines. IMPORTANCE Neisseria meningitidis outer membrane vesicles (OMV) are a component of the capsular group B meningococcal vaccine 4CMenB (Bexsero) and have been shown to induce 30% efficacy against gonococcal infection. They are composed of multiple antigens and are considered an interesting delivery platform for vaccines against several bacterial diseases. However, the protective antibody response after two or three doses of OMV-based meningococcal vaccines appears short-lived. We explored the B cell response induced to a dominant and a subdominant antigen in a meningococcal OMV vaccine in a clinical trial and showed that immune responses are elicited to minor antigens. However, memory B cell responses to the OMV were low or absent and mainly detected in participants who had evidence of preexisting immunity against the antigens. Failure to induce a strong B cell response may be linked with the low persistence of protective responses.
Keywords: Neisseria; bacteria; genetic modification; infection; meningitidis; meningitis; meningococcal; outer membrane; outer membrane proteins; outer membrane vesicles; vaccine; vesicles.
Conflict of interest statement
The authors declare a conflict of interest. A.J.P. is Chair of UK Dept. Health and Social Care's (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) and is a member of the WHO's SAGE. A.J.P. is an NIHR Senior Investigator. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, NIHR, or WHO. M.S. is supported via salary awards from the BC Children's Hospital Foundation, the Canadian Child Health Clinician Scientist Program, and the Michael Smith Foundation for Health Research. M.S. has been an investigator on projects funded by Pfizer, Merck, Seqirus, Sanofi-Pasteur, VBI Vaccines, and GlaxoSmithKline. All funds have been paid to his institute, and he has not received any personal payments. Through the Consulting Services of Oxford University Innovation, M.C.J.M. undertakes occasional consultancy work for Pfizer, GSK, and Novartis. J.D.’s laboratory has received funding from GlaxoSmithKline for vaccine-related research. I.M.F. was an employee at NIBSC, a centre of the Medicines and Healthcare products Regulatory Agency. The clinical trial was approved by the MHRA prior to the merger of NIBSC with the agency. G.N. is currently an employee of Vaccibody. A.J.P., C.S.R., L.M., and C.D. are inventors on a patent in the field of meningococcal vaccines. A.J.P. waives all his rights on any patent. C.A.G. and A.L. declare no conflict. The views expressed in this publication are those of the authors.
Figures

Similar articles
-
Identification of immunogenic outer membrane vesicle vaccine antigen components using a meningococcal protein microarray.Vaccine. 2025 Apr 19;53:126953. doi: 10.1016/j.vaccine.2025.126953. Epub 2025 Mar 4. Vaccine. 2025. PMID: 40043411
-
A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial.J Infect. 2015 Sep;71(3):326-37. doi: 10.1016/j.jinf.2015.05.006. Epub 2015 May 15. J Infect. 2015. PMID: 25982025 Free PMC article. Clinical Trial.
-
Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870.J Infect Dis. 2005 Aug 15;192(4):580-90. doi: 10.1086/432102. Epub 2005 Jul 15. J Infect Dis. 2005. PMID: 16028126 Free PMC article.
-
Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis.Vaccine. 2009 Jun 24;27 Suppl 2:B3-12. doi: 10.1016/j.vaccine.2009.04.071. Epub 2009 May 28. Vaccine. 2009. PMID: 19481313 Review.
-
Outer membrane vesicle vaccines.Semin Immunol. 2020 Aug;50:101433. doi: 10.1016/j.smim.2020.101433. Epub 2020 Dec 9. Semin Immunol. 2020. PMID: 33309166 Review.
Cited by
-
Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential.Nano Converg. 2024 Jul 11;11(1):28. doi: 10.1186/s40580-024-00434-5. Nano Converg. 2024. PMID: 38990415 Free PMC article. Review.
-
Application of a Neisseria meningitidis antigen microarray to identify candidate vaccine proteins from a human Phase I clinical trial.Vaccine. 2022 Jun 21;40(28):3835-3842. doi: 10.1016/j.vaccine.2022.05.032. Epub 2022 May 21. Vaccine. 2022. PMID: 35610106 Free PMC article. Clinical Trial.
-
Extracellular Vesicles: Recent Insights Into the Interaction Between Host and Pathogenic Bacteria.Front Immunol. 2022 May 25;13:840550. doi: 10.3389/fimmu.2022.840550. eCollection 2022. Front Immunol. 2022. PMID: 35693784 Free PMC article. Review.
-
Outer-Membrane Vesicles of Fusobacterium necrophorum: A Proteomic, Lipidomic, and Functional Characterization.Microorganisms. 2023 Aug 14;11(8):2082. doi: 10.3390/microorganisms11082082. Microorganisms. 2023. PMID: 37630642 Free PMC article.
-
Amplification of microbial DNA from bacterial extracellular vesicles from human placenta.Front Microbiol. 2023 Jul 13;14:1213234. doi: 10.3389/fmicb.2023.1213234. eCollection 2023. Front Microbiol. 2023. PMID: 37520380 Free PMC article.
References
-
- Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, et al. . 2018. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Heal https://www.sciencedirect.com/science/article/pii/S2352464218301032?via%.... - PubMed
-
- Masforrol Y, Gil J, García D, Noda J, Ramos Y, Betancourt L, Guirola O, González S, Acevedo B, Besada V, Reyes O, González LJ. 2017. A deeper mining on the protein composition of VA-MENGOC-BC: an OMV-based vaccine against N. meningitidis serogroup B and C. Hum Vaccin Immunother 13:2548–2560. doi:10.1080/21645515.2017.1356961. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

