Glutathione Is Involved in the Reduction of Methylarsenate to Generate Antibiotic Methylarsenite in Enterobacter sp. Strain CZ-1
- PMID: 35080903
- PMCID: PMC8939317
- DOI: 10.1128/aem.02467-21
Glutathione Is Involved in the Reduction of Methylarsenate to Generate Antibiotic Methylarsenite in Enterobacter sp. Strain CZ-1
Abstract
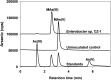
Methylarsenate (MAs(V)) is a product of microbial arsenic (As) biomethylation and has also been widely used as an herbicide. Some microbes are able to reduce nontoxic MAs(V) to highly toxic methylarsenite (MAs(III)) possibly as an antibiotic. The mechanism of MAs(V) reduction in microbes has not been elucidated. Here, we found that the bacterium Enterobacter sp. CZ-1 isolated from an As-contaminated paddy soil has a strong ability to reduce MAs(V) to MAs(III). Using a MAs(III)-responsive biosensor to detect MAs(V) reduction in E. coli Trans5α transformants of a genomic library of Enterobacter sp. CZ-1, we identified gshA, encoding a glutamate-cysteine ligase, as a key gene involved in MAs(V) reduction. Heterologous expression of gshA increased the biosynthesis of glutathione (GSH) and MAs(V) reduction in E. coli Trans5α. Deletion of gshA in Enterobacter sp. CZ-1 abolished its ability to synthesize GSH and decreased its MAs(V) reduction ability markedly, which could be restored by supplementation of exogenous GSH. In the presence of MAs(V), Enterobacter sp. CZ-1 was able to inhibit the growth of Bacillus subtilis 168; this ability was lost in the gshA-deleted mutant. In addition, deletion of gshA greatly decreased the reduction of arsenate to arsenite. These results indicate that GSH plays an important role in MAs(V) reduction to generate MAs(III) as an antibiotic. IMPORTANCE Arsenic is a ubiquitous environmental toxin. Some microbes detoxify inorganic arsenic through biomethylation, generating relatively nontoxic pentavalent methylated arsenicals, such as methylarsenate. Methylarsenate has also been widely used as an herbicide. Surprisingly, some microbes reduce methylarsenate to highly toxic methylarsenite possibly to use the latter as an antibiotic. How microbes reduce methylarsenate to methylarsenite is unknown. Here, we show that gshA encoding a glutamate-cysteine ligase in the glutathione biosynthesis pathway is involved in methylarsenate reduction in Enterobacter sp. CZ-1. Our study provides new insights into the crucial role of glutathione in the transformation of a common arsenic compound to a natural antibiotic.
Keywords: Enterobacter sp. CZ-1; antibiotic; arsenic; glutathione; gshA; methylarsenate; methylarsenite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

