Distinct Metabolic Flow in Response to Temperature in Thermotolerant Kluyveromyces marxianus
- PMID: 35080905
- PMCID: PMC8939337
- DOI: 10.1128/AEM.02006-21
Distinct Metabolic Flow in Response to Temperature in Thermotolerant Kluyveromyces marxianus
Abstract
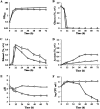
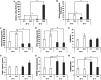
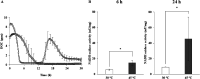
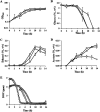
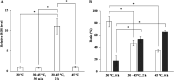
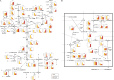
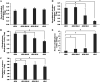
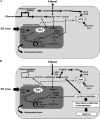
The intrinsic mechanism of the thermotolerance of Kluyveromyces marxianus was investigated by comparison of its physiological and metabolic properties at high and low temperatures. After glucose consumption, the conversion of ethanol to acetic acid became gradually prominent only at a high temperature (45°C) and eventually caused a decline in viability, which was prevented by exogenous glutathione. Distinct levels of reactive oxygen species (ROS), glutathione, and NADPH suggest a greater accumulation of ROS and enhanced ROS-scavenging activity at a high temperature. Fusion and fission forms of mitochondria were dominantly observed at 30°C and 45°C, respectively. Consistent results were obtained by temperature upshift experiments, including transcriptomic and enzymatic analyses, suggesting a change of metabolic flow from glycolysis to the pentose phosphate pathway. The results of this study suggest that K. marxianus survives at a high temperature by scavenging ROS via metabolic change for a period until a critical concentration of acetate is reached. IMPORTANCE Kluyveromyces marxianus, a thermotolerant yeast, can grow well at temperatures over 45°C, unlike Kluyveromyces lactis, which belongs to the same genus, or Saccharomyces cerevisiae, which is a closely related yeast. K. marxianus may thus bear an intrinsic mechanism to survive at high temperatures. This study revealed the thermotolerant mechanism of the yeast, including ROS scavenging with NADPH, which is generated by changes in metabolic flow.
Keywords: Kluyveromyces marxianus; NADPH; acetic acid; reactive oxygen species; thermotolerance; thermotolerant yeast; transcriptome analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Understanding the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation based on integration of RNA-Seq and metabolite data.Appl Microbiol Biotechnol. 2019 Mar;103(6):2715-2729. doi: 10.1007/s00253-019-09637-x. Epub 2019 Jan 23. Appl Microbiol Biotechnol. 2019. PMID: 30673809
-
Reconstruction and analysis of a Kluyveromyces marxianus genome-scale metabolic model.BMC Bioinformatics. 2019 Nov 6;20(1):551. doi: 10.1186/s12859-019-3134-5. BMC Bioinformatics. 2019. PMID: 31694544 Free PMC article.
-
Thermotolerant Yeast Kluyveromyces marxianus Reveals More Tolerance to Heat Shock than the Brewery Yeast Saccharomyces cerevisiae.Biocontrol Sci. 2018;23(3):133-138. doi: 10.4265/bio.23.133. Biocontrol Sci. 2018. PMID: 30249963
-
Kluyveromyces marxianus as a host for heterologous protein synthesis.Appl Microbiol Biotechnol. 2016 Jul;100(14):6193-6208. doi: 10.1007/s00253-016-7645-y. Epub 2016 Jun 3. Appl Microbiol Biotechnol. 2016. PMID: 27260286 Review.
-
Stress response and adaptation mechanisms in Kluyveromyces marxianus.Adv Appl Microbiol. 2024;126:27-62. doi: 10.1016/bs.aambs.2024.02.003. Epub 2024 Mar 12. Adv Appl Microbiol. 2024. PMID: 38637106 Review.
Cited by
-
Combined transcriptomics and metabolomics analyses reveal the molecular mechanism of heat tolerance in Pichia kudriavzevii.Front Microbiol. 2025 Apr 9;16:1572004. doi: 10.3389/fmicb.2025.1572004. eCollection 2025. Front Microbiol. 2025. PMID: 40270822 Free PMC article.
-
Advances in stress-tolerance elements for microbial cell factories.Synth Syst Biotechnol. 2024 Jun 28;9(4):793-808. doi: 10.1016/j.synbio.2024.06.008. eCollection 2024 Dec. Synth Syst Biotechnol. 2024. PMID: 39072145 Free PMC article. Review.
-
Comprehensive Response of Rhodosporidium kratochvilovae to Glucose Starvation: A Transcriptomics-Based Analysis.Microorganisms. 2023 Aug 27;11(9):2168. doi: 10.3390/microorganisms11092168. Microorganisms. 2023. PMID: 37764012 Free PMC article.
-
Dissecting an ancient stress resistance trait syndrome in the compost yeast Kluyveromyces marxianus.bioRxiv [Preprint]. 2023 Dec 22:2023.12.21.572915. doi: 10.1101/2023.12.21.572915. bioRxiv. 2023. PMID: 38187519 Free PMC article. Preprint.
-
A genome-informed higher rank classification of the biotechnologically important fungal subphylum Saccharomycotina.Stud Mycol. 2023 Jun;105:1-22. doi: 10.3114/sim.2023.105.01. Epub 2023 May 25. Stud Mycol. 2023. PMID: 38895705 Free PMC article.
References
-
- Rodrussamee N, Lertwattanasakul N, Hirata K, Suprayogi, Limtong S, Kosaka T, Yamada M. 2011. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl Microbiol Biotechnol 90:1573–1586. 10.1007/s00253-011-3218-2. - DOI - PubMed
-
- Lertwattanasakul N, Rodrussamee N, Suprayogi, Limtong S, Thanonkeo P, Kosaka T, Yamada M. 2011. Utilization capability of sucrose, raffinose and inulin and its less-sensitiveness to glucose repression in thermotolerant yeast Kluyveromyces marxianus DMKU 3-1042. AMB Express 1:20. 10.1186/2191-0855-1-20. - DOI - PMC - PubMed
-
- Banat IM, Nigam P, Singh D, Marchant R, McHale AP. 1998. Ethanol production at elevated temperatures and alcohol concentrations. I. Yeasts in general. World J Microbiol Biotechnol 14:809–821. 10.1023/A:1008802704374. - DOI
-
- Murata M, Nitiyon S, Lertwattanasakul N, Sootsuwan K, Kosaka T, Thanonkeo P, Limtong S, Yamada M. 2015. High-temperature fermentation technology for low-cost bioethanol. J Jpn Inst Energy 94:1154–1162. 10.3775/jie.94.1154. - DOI
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

