Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy
- PMID: 35080914
- PMCID: PMC9014398
- DOI: 10.1126/scitranslmed.abl3836
Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy
Abstract
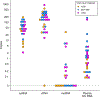
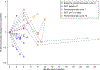
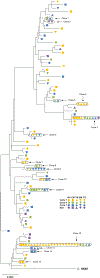
In people living with HIV (PLWH) on antiretroviral therapy (ART), virus persists in a latent form where there is minimal transcription or protein expression. Latently infected cells are a major barrier to curing HIV. Increasing HIV transcription and viral production in latently infected cells could facilitate immune recognition and reduce the pool of infected cells that persist on ART. Given that programmed cell death protein 1 (PD-1) expressing CD4+ T cells are preferentially infected with HIV in PLWH on ART, we aimed to determine whether administration of antibodies targeting PD-1 would reverse HIV latency in vivo. We therefore evaluated the impact of intravenous administration of pembrolizumab every 3 weeks on HIV latency in 32 PLWH and cancer on ART. After the first infusion of anti-PD-1, we observed a median 1.32-fold increase in unspliced HIV RNA and 1.61-fold increase in unspliced RNA:DNA ratio in sorted blood CD4+ T cells compared to baseline. We also observed a 1.65-fold increase in plasma HIV RNA. The frequency of CD4+ T cells with inducible virus evaluated using the tat/rev limiting dilution assay was higher after 6 cycles compared to baseline. Phylogenetic analyses of HIV env sequences in a participant who developed low concentrations of HIV viremia after 6 cycles of pembrolizumab did not demonstrate clonal expansion of HIV-infected cells. These data are consistent with anti-PD-1 being able to reverse HIV latency in vivo and support the rationale for combining anti-PD-1 with other interventions to reduce the HIV reservoir.
Trial registration: ClinicalTrials.gov NCT02595866.
Conflict of interest statement
Figures

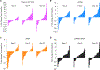
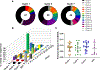
References
-
- Dybul M, Attoye T, Baptiste S, Cherutich P, Dabis F, Deeks SG, Dieffenbach C, Doehle B, Goodenow MM, Jiang A, Kemps D, Lewin SR, Lumpkin MM, Mathae L, McCune JM, Ndung’u T, Nsubuga M, Peay HL, Pottage J, Warren M, Sikazwe I; Sunnylands 2019 Working Group, The case for an HIV cure and how to get there. Lancet HIV 8, e51–e58 (2021). - PMC - PubMed
-
- Simonetti FR, Zhang H, Soroosh GP, Duan J, Rhodehouse K, Hill AL, Beg SA, McCormick K, Raymond HE, Nobles CL, Everett JK, Kwon KJ, White JA, Lai J, Margolick JB, Hoh R, Deeks SG, Bushman FD, Siliciano JD, Siliciano RF, Antigen-driven clonal selection shapes the persistence of HIV-1-infected CD4+ T cells in vivo. J. Clin. Invest 131, e145254 (2021). - PMC - PubMed
-
- Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E, HIV-1 remission following CCR5∆32/∆32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

