In silico, in vitro and in vivo safety evaluation of Limosilactobacillus reuteri strains ATCC PTA-126787 & ATCC PTA-126788 for potential probiotic applications
- PMID: 35081129
- PMCID: PMC8791467
- DOI: 10.1371/journal.pone.0262663
In silico, in vitro and in vivo safety evaluation of Limosilactobacillus reuteri strains ATCC PTA-126787 & ATCC PTA-126788 for potential probiotic applications
Abstract
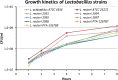
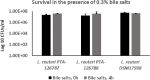
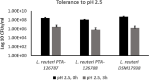
The last two decades have witnessed a tremendous growth in probiotics and in the numbers of publications on their potential health benefits. Owing to their distinguishing beneficial effects and long history of safe use, species belonging to the Lactobacillus genus are among the most widely used probiotic species in human food and dietary supplements and are finding increased use in animal feed. Here, we isolated, identified, and evaluated the safety of two novel Limosilactobacillus reuteri (L. reuteri) isolates, ATCC PTA-126787 & ATCC PTA-126788. More specifically, we sequenced the genomes of these two L. reuteri strains using the PacBio sequencing platform. Using a combination of biochemical and genetic methods, we identified the two strains as belonging to L. reuteri species. Detailed in silico analyses showed that the two strains do not encode for any known genetic sequences of concern for human or animal health. In vitro assays confirmed that the strains are susceptible to clinically relevant antibiotics and do not produce potentially harmful by-products such as biogenic amines. In vitro bile and acid tolerance studies demonstrated that the two strains have similar survival profiles as the commercial L. reuteri probiotic strain DSM 17938. Most importantly, daily administration of the two probiotic strains to broiler chickens in drinking water for 26 days did not induce any adverse effect, clinical disease, or histopathological lesions, supporting the safety of the strains in an in vivo avian model. All together, these data provide in silico, in vitro and in vivo evidence of the safety of the two novel candidates for potential probiotic applications in humans as well as animals.
Conflict of interest statement
I have read the journal’s policy and want to declare the following conflicts of interest. The authors DG, SPM, NRT, VR, AV, DS, MP, AA, AK, EBH are employees of Elanco Animal Health, Inc. The author NL was an employee of Elanco Animal Health, Inc. at the time the work was done. Elanco Animal Health, Inc. is a company that develops, manufactures, and sells veterinary pharmaceuticals and nutritionals. This affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





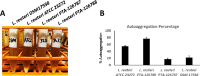

References
-
- FAO/WHO. Guidelines for the evaluation of probiotics in food report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. London Ontario, Canada: World Health Organization. 2002.
-
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al.. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. doi: 10.1038/nrgastro.2014.66 - DOI - PubMed
-
- Zoumpopoulou G, Tzouvanou A, Mavrogonatou E, Alexandraki V, Georgalaki M, Anastasiou R, et al.. Probiotic features of lactic acid bacteria isolated from a diverse pool of traditional greek dairy products regarding specific strain-host interactions. Probiotics Antimicrob Proteins. 2018;10(2):313–22. doi: 10.1007/s12602-017-9311-9 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

