Proline-specific aminopeptidase P prevents replication-associated genome instability
- PMID: 35081133
- PMCID: PMC8820600
- DOI: 10.1371/journal.pgen.1010025
Proline-specific aminopeptidase P prevents replication-associated genome instability
Abstract
Genotoxic stress during DNA replication constitutes a serious threat to genome integrity and causes human diseases. Defects at different steps of DNA metabolism are known to induce replication stress, but the contribution of other aspects of cellular metabolism is less understood. We show that aminopeptidase P (APP1), a metalloprotease involved in the catabolism of peptides containing proline residues near their N-terminus, prevents replication-associated genome instability. Functional analysis of C. elegans mutants lacking APP-1 demonstrates that germ cells display replication defects including reduced proliferation, cell cycle arrest, and accumulation of mitotic DSBs. Despite these defects, app-1 mutants are competent in repairing DSBs induced by gamma irradiation, as well as SPO-11-dependent DSBs that initiate meiotic recombination. Moreover, in the absence of SPO-11, spontaneous DSBs arising in app-1 mutants are repaired as inter-homologue crossover events during meiosis, confirming that APP-1 is not required for homologous recombination. Thus, APP-1 prevents replication stress without having an apparent role in DSB repair. Depletion of APP1 (XPNPEP1) also causes DSB accumulation in mitotically-proliferating human cells, suggesting that APP1's role in genome stability is evolutionarily conserved. Our findings uncover an unexpected role for APP1 in genome stability, suggesting functional connections between aminopeptidase-mediated protein catabolism and DNA replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



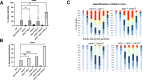

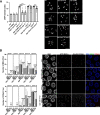

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

