New insights into the role of endosomal proteins for African swine fever virus infection
- PMID: 35081156
- PMCID: PMC8820605
- DOI: 10.1371/journal.ppat.1009784
New insights into the role of endosomal proteins for African swine fever virus infection
Abstract
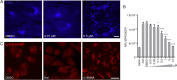
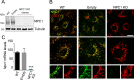
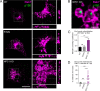
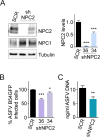
African swine fever virus (ASFV) infectious cycle starts with the viral adsorption and entry into the host cell. Then, the virus is internalized via clathrin/dynamin mediated endocytosis and macropinocytosis. Similar to other viruses, ASF virion is then internalized and incorporated into the endocytic pathway. While the endosomal maturation entails luminal acidification, the decrease in pH acts on the multilayer structure of the virion dissolving the outer capsid. Upon decapsidation, the inner viral membrane is exposed to interact with the limiting membrane of the late endosome for fusion. Viral fusion is then necessary for the egress of incoming virions from endosomes into the cytoplasm, however this remains an intriguing and yet essential process for infection, specifically for the egress of viral nucleic acid into the cytoplasm for replication. ASFV proteins E248R and E199L, located at the exposed inner viral membrane, might be implicated in the fusion step. An interaction between these viral proteins and cellular endosomal proteins such as the Niemann-Pick C type 1 (NPC1) and lysosomal membrane proteins (Lamp-1 and -2) was shown. Furthermore, the silencing of these proteins impaired ASFV infection. It was also observed that NPC1 knock-out cells using CRISPR jeopardized ASFV infection and that the progression and endosomal exit of viral cores was arrested within endosomes at viral entry. These results suggest that the interactions of ASFV proteins with some endosomal proteins might be important for the membrane fusion step. In addition to this, reductions on ASFV infectivity and replication in NPC1 KO cells were accompanied by fewer and smaller viral factories. Our findings pave the way to understanding the role of proteins of the endosomal membrane in ASFV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Andres G, Charro D, Matamoros T, Dillard RS, Abrescia NGA. The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. J Biol Chem. 2020;295(1):1–12. Epub 2019/10/28. doi: 10.1074/jbc.AC119.011196 ; PubMed Central PMCID: PMC6952596. - DOI - PMC - PubMed
-
- Brookes V, Barrett T, Ward M, Roby J, Hernandez-Jover M, Cross E, et al.. A scoping review of African swine fever virus spread between domestic and free-living pigs. Transboundary and Emerging Diseases. 2020. - PubMed

