Homologous and Heterologous Covid-19 Booster Vaccinations
- PMID: 35081293
- PMCID: PMC8820244
- DOI: 10.1056/NEJMoa2116414
Homologous and Heterologous Covid-19 Booster Vaccinations
Abstract
Background: Although the three vaccines against coronavirus disease 2019 (Covid-19) that have received emergency use authorization in the United States are highly effective, breakthrough infections are occurring. Data are needed on the serial use of homologous boosters (same as the primary vaccine) and heterologous boosters (different from the primary vaccine) in fully vaccinated recipients.
Methods: In this phase 1-2, open-label clinical trial conducted at 10 sites in the United States, adults who had completed a Covid-19 vaccine regimen at least 12 weeks earlier and had no reported history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection received a booster injection with one of three vaccines: mRNA-1273 (Moderna) at a dose of 100 μg, Ad26.COV2.S (Johnson & Johnson-Janssen) at a dose of 5×1010 virus particles, or BNT162b2 (Pfizer-BioNTech) at a dose of 30 μg. The primary end points were safety, reactogenicity, and humoral immunogenicity on trial days 15 and 29.
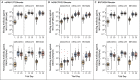
Results: Of the 458 participants who were enrolled in the trial, 154 received mRNA-1273, 150 received Ad26.COV2.S, and 153 received BNT162b2 as booster vaccines; 1 participant did not receive the assigned vaccine. Reactogenicity was similar to that reported for the primary series. More than half the recipients reported having injection-site pain, malaise, headache, or myalgia. For all combinations, antibody neutralizing titers against a SARS-CoV-2 D614G pseudovirus increased by a factor of 4 to 73, and binding titers increased by a factor of 5 to 55. Homologous boosters increased neutralizing antibody titers by a factor of 4 to 20, whereas heterologous boosters increased titers by a factor of 6 to 73. Spike-specific T-cell responses increased in all but the homologous Ad26.COV2.S-boosted subgroup. CD8+ T-cell levels were more durable in the Ad26.COV2.S-primed recipients, and heterologous boosting with the Ad26.COV2.S vaccine substantially increased spike-specific CD8+ T cells in the mRNA vaccine recipients.
Conclusions: Homologous and heterologous booster vaccines had an acceptable safety profile and were immunogenic in adults who had completed a primary Covid-19 vaccine regimen at least 12 weeks earlier. (Funded by the National Institute of Allergy and Infectious Diseases; DMID 21-0012 ClinicalTrials.gov number, NCT04889209.).
Copyright © 2022 Massachusetts Medical Society.
Figures


References
-
- Centers for Disease Control and Prevention. COVID data tracker: COVID-19 vaccinations in the United States (https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-...).
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- UM1AI148450/National Institute of Allergy and Infectious Diseases
- UM1 AI148372/AI/NIAID NIH HHS/United States
- UM1AI148574/National Institute of Allergy and Infectious Diseases
- UM1AI148575/National Institute of Allergy and Infectious Diseases
- UM1 AI148452/AI/NIAID NIH HHS/United States
- 75N93019C00050/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- UM1AI148576/National Institute of Allergy and Infectious Diseases
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- T32 AI074492/AI/NIAID NIH HHS/United States
- UM1AI148452/National Institute of Allergy and Infectious Diseases
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1AI148373/National Institute of Allergy and Infectious Diseases
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1AI148689/National Institute of Allergy and Infectious Diseases
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1AI148684/National Institute of Allergy and Infectious Diseases
- UM1 AI148574/AI/NIAID NIH HHS/United States
- UM1AI148573/National Institute of Allergy and Infectious Diseases
- UM1AI148372/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
