Soluble urokinase plasminogen activator receptor levels are predictive of COVID-19 severity in Afro-Caribbean patients
- PMID: 35081737
- PMCID: PMC8809376
- DOI: 10.2217/bmm-2021-0669
Soluble urokinase plasminogen activator receptor levels are predictive of COVID-19 severity in Afro-Caribbean patients
Abstract
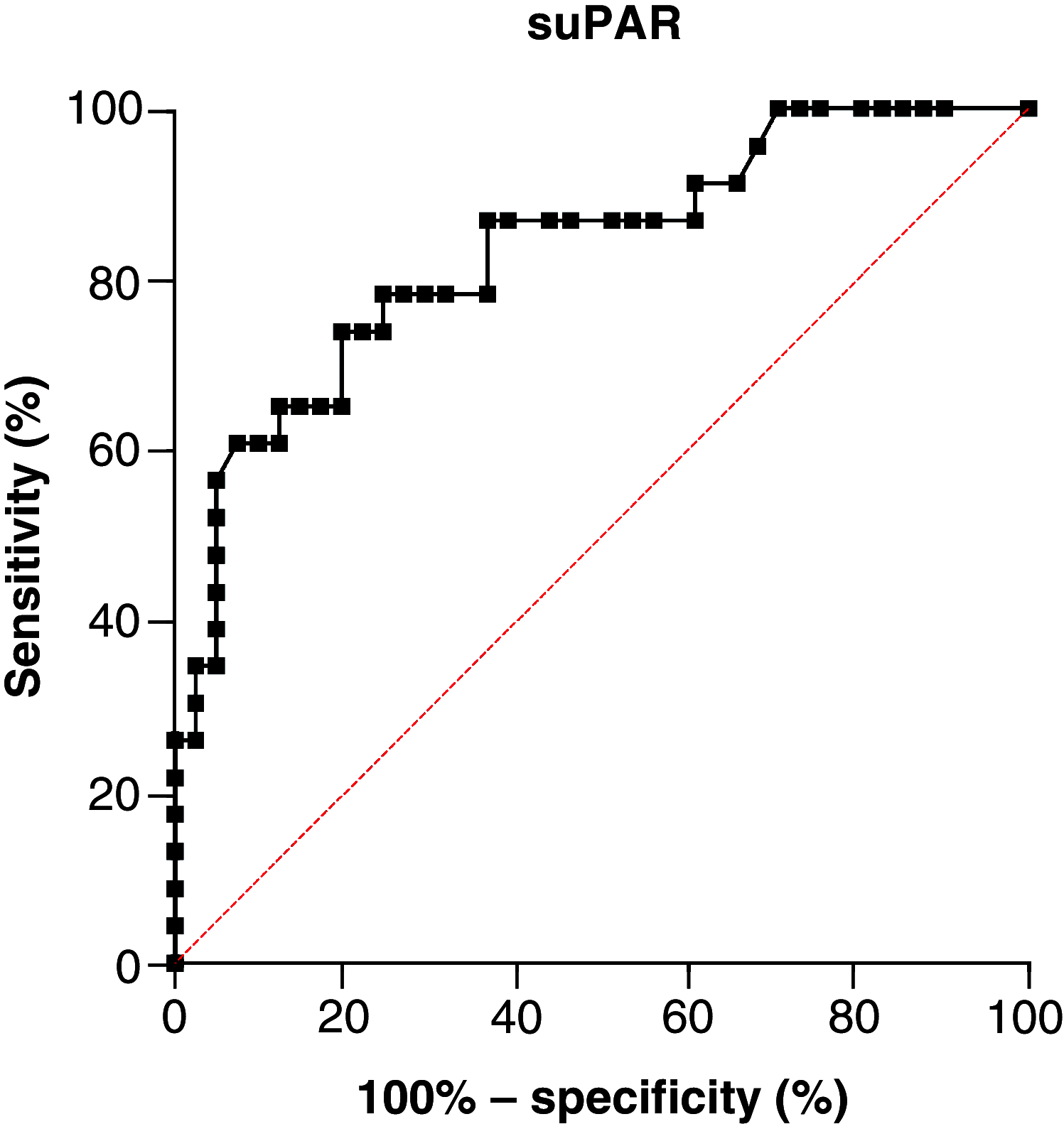
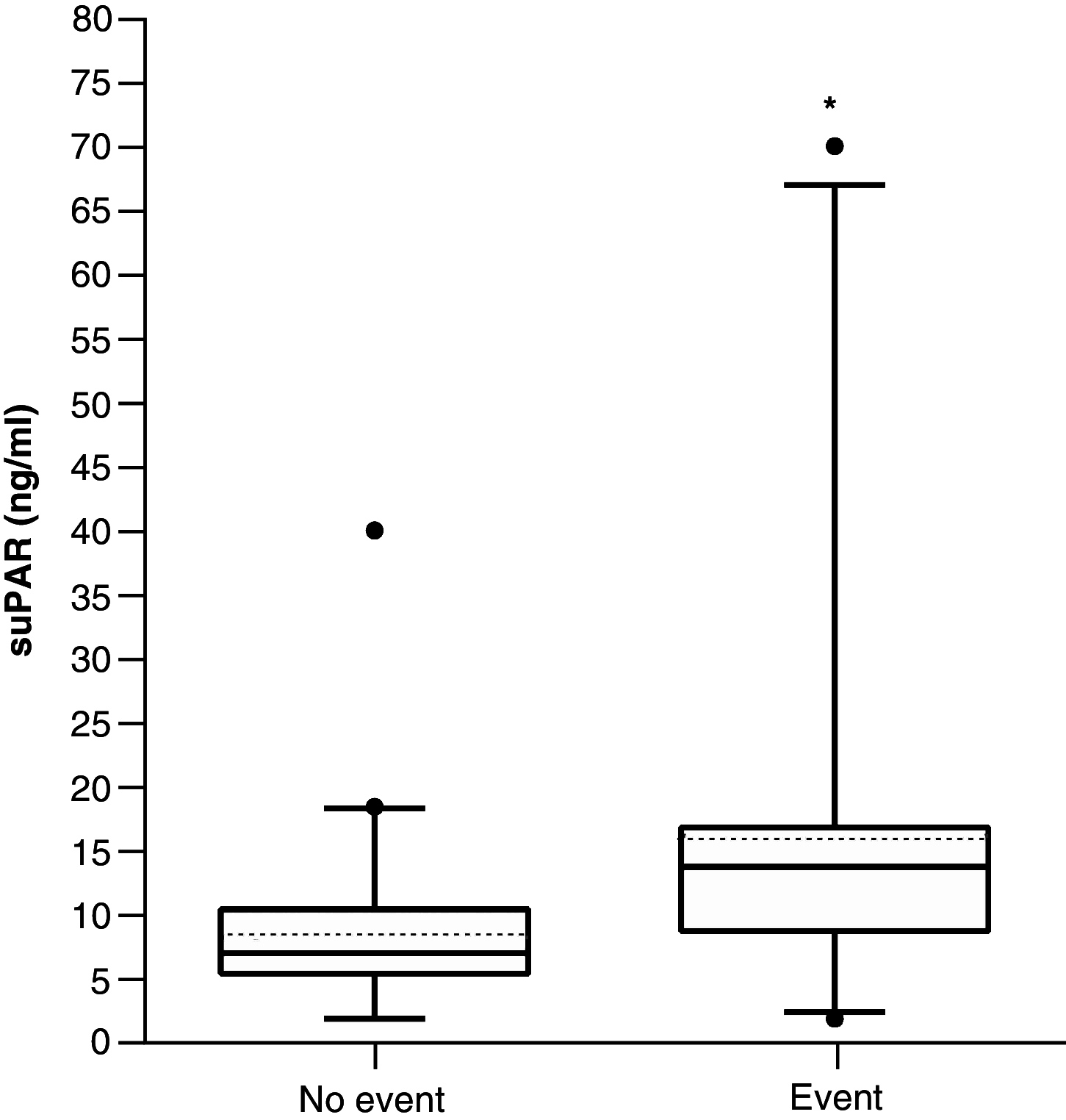
Aim: To investigate association between soluble urokinase plasminogen activator receptor (suPAR) plasma levels at admission and incidence of complications in COVID-19 patients. Patients & methods: We considered Afro-Caribbean patients (n = 64) admitted to the hospital between 1 February 2020 and 28 February 2021. Primary outcome was time from the hospital admission until intensive care unit care or death. Results: Primary outcome (hazard ratio, HR [95%CI]) was associated with higher CT scan severity score (3.18 [1.15-8.78], p = 0.025), National Early Warning Score (NEWS2; 1.43 [1.02-2.02], p = 0.041) and suPAR (1.28 [1.06-2.06], p = 0.041). Kaplan-Meier analysis indicated patients with suPAR level above 8.95 ng/ml had a worse outcome (7.95 [3.33-18.97], p < 0.001). Conclusion: Our study suggests that COVID-19 patients with increased baseline suPAR levels are at a high risk of complications.
Keywords: Afro-Caribbean; COVID-19; National Early Warning Score; chest CT severity score; intensive care; soluble urokinase plasminogen activator receptor.
Plain language summary
Plain language summary Our aim was to investigate association between the plasma levels of soluble urokinase plasminogen activator receptor (suPAR) at admission and incidence of complications in COVID-19 patients. Increased suPAR level has been previously associated with activation of inflammation and coagulation, which important features of COVID-19. We considered Afro-Caribbean patients admitted to the hospital between 1 February 2020 and 28 February 2021. Primary outcome was time from the hospital admission until intensive care unit care or death. The use of an integrative prediction tool which combines simple clinical score (NEWS2), imaging technique (chest CT severity score) and suPAR plasma levels has potent predictive value for COVID-19 outcome.
Figures

References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of COVID-19 (COVID-19): a review. JAMA 324(8), 782–793 (2020). - PubMed
-
•• Provides information on epidemiological, clinical and laboratory characteristics of COVID-19.
-
- Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N. Engl. J. Med. 383(25), 2451–2460 (2020). - PubMed
-
•• Provides information on the evaluation and management of severe COVID-19.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
