Surface NMR using quantum sensors in diamond
- PMID: 35082146
- PMCID: PMC8812553
- DOI: 10.1073/pnas.2111607119
Surface NMR using quantum sensors in diamond
Abstract
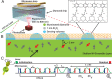
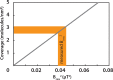
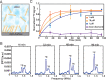
NMR is a noninvasive, molecular-level spectroscopic technique widely used for chemical characterization. However, it lacks the sensitivity to probe the small number of spins at surfaces and interfaces. Here, we use nitrogen vacancy (NV) centers in diamond as quantum sensors to optically detect NMR signals from chemically modified thin films. To demonstrate the method's capabilities, aluminum oxide layers, common supports in catalysis and materials science, are prepared by atomic layer deposition and are subsequently functionalized by phosphonate chemistry to form self-assembled monolayers. The surface NV-NMR technique detects spatially resolved NMR signals from the monolayer, indicates chemical binding, and quantifies molecular coverage. In addition, it can monitor in real time the formation kinetics at the solid-liquid interface. With our approach, we show that NV quantum sensors are a surface-sensitive NMR tool with femtomole sensitivity for in situ analysis in catalysis, materials, and biological research.
Keywords: NV center in diamond; quantum sensing; self-assembled monolayer; spectroscopy; surface analysis.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Salmeron M., From surfaces to interfaces: Ambient pressure XPS and beyond. Top. Catal. 61, 2044–2051 (2018).
-
- Velasco-Vélez J. J., et al. , Atmospheric pressure X-ray photoelectron spectroscopy apparatus: Bridging the pressure gap. Rev. Sci. Instrum. 87, 053121 (2016). - PubMed
-
- Bolli E., Kaciulis S., Mezzi A., ESCA as a tool for exploration of metals’ surface. Coatings 10, 1182 (2020).

