Immune-checkpoint inhibitors: long-term implications of toxicity
- PMID: 35082367
- PMCID: PMC8790946
- DOI: 10.1038/s41571-022-00600-w
Immune-checkpoint inhibitors: long-term implications of toxicity
Abstract
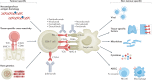
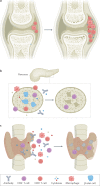
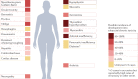
The development of immune-checkpoint inhibitors (ICIs) has heralded a new era in cancer treatment, enabling the possibility of long-term survival in patients with metastatic disease, and providing new therapeutic indications in earlier-stage settings. As such, characterizing the long-term implications of receiving ICIs has grown in importance. An abundance of evidence exists describing the acute clinical toxicities of these agents, although chronic effects have not been as well catalogued. Nonetheless, emerging evidence indicates that persistent toxicities might be more common than initially suggested. While generally low-grade, these chronic sequelae can affect the endocrine, rheumatological, pulmonary, neurological and other organ systems. Fatal toxicities also comprise a diverse set of clinical manifestations and can occur in 0.4-1.2% of patients. This risk is a particularly relevant consideration in light of the possibility of long-term survival. Finally, the effects of immune-checkpoint blockade on a diverse range of immune processes, including atherosclerosis, heart failure, neuroinflammation, obesity and hypertension, have not been characterized but remain an important area of research with potential relevance to cancer survivors. In this Review, we describe the current evidence for chronic immune toxicities and the long-term implications of these effects for patients receiving ICIs.
© 2022. Springer Nature Limited.
Conflict of interest statement
D.B.J. has acted as a consultant and/or advisor for BMS, Catalyst, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer and Targovax, and receives research funding from BMS and Incyte. D.B.J. and J.J.M. have a patent pending for use of abatacept to reverse ICI toxicities. D.B.J. and J.M.B. have a patent pending for use of MHC II as a biomarker for ICI response. J.M.B. receives research funding from Genentech and Incyte. C.A.N. and J.J.M. declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

