Recent Advances in Modeling Mitochondrial Cardiomyopathy Using Human Induced Pluripotent Stem Cells
- PMID: 35083221
- PMCID: PMC8784695
- DOI: 10.3389/fcell.2021.800529
Recent Advances in Modeling Mitochondrial Cardiomyopathy Using Human Induced Pluripotent Stem Cells
Abstract
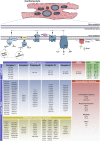
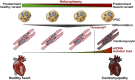
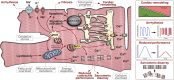
Around one third of patients with mitochondrial disorders develop a kind of cardiomyopathy. In these cases, severity is quite variable ranging from asymptomatic status to severe manifestations including heart failure, arrhythmias, and sudden cardiac death. ATP is primarily generated in the mitochondrial respiratory chain via oxidative phosphorylation by utilizing fatty acids and carbohydrates. Genes in both the nuclear and the mitochondrial DNA encode components of this metabolic route and, although mutations in these genes are extremely rare, the risk to develop cardiac symptoms is significantly higher in this patient cohort. Additionally, infants with cardiovascular compromise in mitochondrial deficiency display a worse late survival compared to patients without cardiac symptoms. At this point, the mechanisms behind cardiac disease progression related to mitochondrial gene mutations are poorly understood and current therapies are unable to substantially restore the cardiac performance and to reduce the disease burden. Therefore, new strategies are needed to uncover the pathophysiological mechanisms and to identify new therapeutic options for mitochondrial cardiomyopathies. Here, human induced pluripotent stem cell (iPSC) technology has emerged to provide a suitable patient-specific model system by recapitulating major characteristics of the disease in vitro, as well as to offer a powerful platform for pre-clinical drug development and for the testing of novel therapeutic options. In the present review, we summarize recent advances in iPSC-based disease modeling of mitochondrial cardiomyopathies and explore the patho-mechanistic insights as well as new therapeutic approaches that were uncovered with this experimental platform. Further, we discuss the challenges and limitations of this technology and provide an overview of the latest techniques to promote metabolic and functional maturation of iPSC-derived cardiomyocytes that might be necessary for modeling of mitochondrial disorders.
Keywords: heteroplasmy; iPSC-derived cardiomyocytes; induced pluripotent stem cells (hiPSCs); mitochondrial cardiomyopathy; mitochondrial disease; mtDNA.
Copyright © 2022 Pavez-Giani and Cyganek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
How can we use stem cell-derived cardiomyocytes to understand the involvement of energetic metabolism in alterations of cardiac function?Front Mol Med. 2023 Sep 1;3:1222986. doi: 10.3389/fmmed.2023.1222986. eCollection 2023. Front Mol Med. 2023. PMID: 39086669 Free PMC article. Review.
-
Disease Modeling of Mitochondrial Cardiomyopathy Using Patient-Specific Induced Pluripotent Stem Cells.Biology (Basel). 2021 Sep 29;10(10):981. doi: 10.3390/biology10100981. Biology (Basel). 2021. PMID: 34681080 Free PMC article. Review.
-
Disease modeling of desmosome-related cardiomyopathy using induced pluripotent stem cell-derived cardiomyocytes.World J Stem Cells. 2023 Mar 26;15(3):71-82. doi: 10.4252/wjsc.v15.i3.71. World J Stem Cells. 2023. PMID: 37007457 Free PMC article. Review.
-
Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system.Acta Biomater. 2020 Jan 15;102:273-286. doi: 10.1016/j.actbio.2019.11.044. Epub 2019 Nov 26. Acta Biomater. 2020. PMID: 31778832
-
Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies.Cardiovasc Res. 2021 Feb 22;117(3):694-711. doi: 10.1093/cvr/cvaa125. Cardiovasc Res. 2021. PMID: 32365198 Free PMC article. Review.
Cited by
-
How can we use stem cell-derived cardiomyocytes to understand the involvement of energetic metabolism in alterations of cardiac function?Front Mol Med. 2023 Sep 1;3:1222986. doi: 10.3389/fmmed.2023.1222986. eCollection 2023. Front Mol Med. 2023. PMID: 39086669 Free PMC article. Review.
-
Application of hiPSC as a Drug Tester Via Mimicking a Personalized Mini Heart.Front Genet. 2022 Apr 14;13:891159. doi: 10.3389/fgene.2022.891159. eCollection 2022. Front Genet. 2022. PMID: 35495144 Free PMC article. Review.
-
Mitochondrial Cardiomyopathy: Molecular Epidemiology, Diagnosis, Models, and Therapeutic Management.Cells. 2022 Nov 6;11(21):3511. doi: 10.3390/cells11213511. Cells. 2022. PMID: 36359908 Free PMC article. Review.
-
Molecular and metabolic landscape of adenosine triphosphate-induced cell death in cardiovascular disease.World J Cardiol. 2024 Dec 26;16(12):689-706. doi: 10.4330/wjc.v16.i12.689. World J Cardiol. 2024. PMID: 39734818 Free PMC article. Review.
-
Advances in human induced pluripotent stem cell (hiPSC)-based disease modelling in cardiogenetics.Med Genet. 2025 Apr 8;37(2):137-146. doi: 10.1515/medgen-2025-2009. eCollection 2025 Jun. Med Genet. 2025. PMID: 40207041 Free PMC article.
References
-
- Bekhite M., González-Delgado A., Hübner S., Haxhikadrija P., Kretzschmar T., Müller T., et al. (2021). The Role of Ceramide Accumulation in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes on Mitochondrial Oxidative Stress and Mitophagy. Free Radic. Biol. Med. 167, 66–80. 10.1016/j.freeradbiomed.2021.02.016 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

