Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries
- PMID: 35083316
- PMCID: PMC8784757
- DOI: 10.1183/23120541.00597-2021
Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries
Abstract
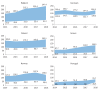
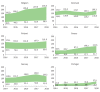
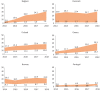
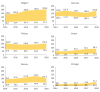
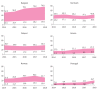
The PERSEIDS study aimed to estimate incidence/prevalence of interstitial lung diseases (ILDs), fibrosing interstitial lung diseases (F-ILDs), idiopathic pulmonary fibrosis (IPF), systemic sclerosis-associated ILD (SSc-ILD), other non-IPF F-ILDs and their progressive-fibrosing (PF) forms in six European countries, as current data are scarce. This retrospective, two-phase study used aggregate data (2014-2018). In Phase 1, incident/prevalent cases of ILDs above were identified from clinical databases through an algorithm based on codes/keywords, and incidence/prevalence was estimated. For non-IPF F-ILDs, the relative percentage of subtypes was also determined. In Phase 2, a subset of non-IPF F-ILD cases was manually reviewed to determine the percentage of PF behaviour and usual interstitial pneumonia-like (UIP-like) pattern. A weighted mean percentage of progression was calculated for each country and used to extrapolate incidence/prevalence of progressive-fibrosing ILDs (PF-ILDs). In 2018, incidence/105 person-years ranged between 9.4 and 83.6 (ILDs), 7.7 and 76.2 (F-ILDs), 0.4 and 10.3 (IPF), 6.6 and 71.7 (non-IPF F-ILDs), and 0.3 and 1.5 (SSc-ILD); and prevalence/105 persons ranged between 33.6 and 247.4 (ILDs), 26.7 and 236.8 (F-ILDs), 2.8 and 31.0 (IPF), 22.3 and 205.8 (non-IPF F-ILDs), and 1.4 and 10.1 (SSc-ILD). Among non-IPF F-ILDs, sarcoidosis was the most frequent subtype. PF behaviour and UIP-like pattern were present in a third of non-IPF F-ILD cases each and hypersensitivity pneumonitis showed the highest percentage of progressive behaviour. Incidence of PF-ILDs ranged between 2.1 and 14.5/105 person-years, and prevalence between 6.9 and 78.0/105 persons. To our knowledge, PERSEIDS is the first study assessing incidence, prevalence and rate of progression of ILDs across several European countries. Still below the threshold for orphan diseases, the estimates obtained were higher and more variable than reported in previous studies, but differences in study design/population must be considered.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: O. Hilberg has nothing to disclose. Conflict of interest: A-M. Hoffmann-Vold had a consultancy and medical writing relationship with Boehringer Ingelheim; received unrestricted grants from Bayer and Boehringer Ingelheim; received consulting fees from Actelion, Boehringer Ingelheim, ARXX and Medscape; received honoraria for lectures and presentations from Actelion, Boehringer Ingelheim, Roche, Merck Sharp & Dohme, Lilly and Medscape; received support for attending meetings and/or travel from Actelion, Boehringer Ingelheim, Roche and Medscape; and was board member of EUSTAR and Nordic PH group. Conflict of interest: V. Smith received grants to research support, as senior clinical investigator from Research Foundation Flanders and Boehringer Ingelheim, research grant from Belgian Fund for Scientific Research in Rheumatic Diseases and educational grant from Janssen-Cilag; received consultancy fees from Boehringer Ingelheim; received honoraria for lectures, presentations, and speaker fees from Accord Healthcare, UCB, Boehringer Ingelheim and Janssen-Cilag; received support for attending meetings and/or travel from Celgene and Boehringer Ingelheim; and was chair (unpaid) to EULAR Study group on Microcirculation in Rheumatic Diseases, co-chair (unpaid) to ACR Study Group on Microcirculation and SCTC working group, and steering committee member (unpaid) to ERN-ReCONNET. Conflict of interest: D. Bouros received consulting fees from Boehringer Ingelheim; received honoraria from Boehringer Ingelheim, Roche and AstraZeneca; received support for attending meetings and/or travel from Boehringer Ingelheim and Roche; and received other financial or nonfinancial interests from Chiesi and ELPEN. Conflict of interest: M. Kilpelainen has nothing to disclose. Conflict of interest: J. Guiot has nothing to disclose. Conflict of interest: A. Morais has nothing to disclose. Conflict of interest: S. Clemente received payment for lectures and presentations and manuscript writing from Boehringer Ingelheim; and was ad hoc expert member of EMEA (January 2020). Conflict of interest: Z. Daniil has nothing to disclose. Conflict of interest: D. Papakosta has nothing to disclose. Conflict of interest: H. Fretheim has nothing to disclose. Conflict of interest: S. Neves has nothing to disclose. Conflict of interest: T.M. Alfaro received consulting fees from Boehringer Ingelheim and Roche; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim and Roche; received support for attending meetings and/or travel from Boehringer Ingelheim and Roche; and participated on a Data Safety Monitoring Board or Advisory Board of Boehringer Ingelheim and Roche. Conflict of interest: K.M. Antoniou received consulting fees from Boehringer Ingelheim and Roche; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim and Roche; received support for attending meetings and/or travel from Boehringer Ingelheim and Roche; and had leadership or fiduciary role in ERS Assembly 12 Secretary (unpaid). Conflict of interest: N. Valveny received funding to TFS for study conduction and medical writing from Boehringer Ingelheim and is employee of TFS. Conflict of interest: G. Asijee is employee of Boehringer Ingelheim. Conflict of interest: S. Soulard is employee of Boehringer Ingelheim. Conflict of interest: W. Wuyts received grants from Boehringer Ingelheim, Roche and Galapagos.
Figures



References
LinkOut - more resources
Full Text Sources
Medical
