Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines Among Incarcerated People in California State Prisons: Retrospective Cohort Study
- PMID: 35083482
- PMCID: PMC8807311
- DOI: 10.1093/cid/ciab1032
Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines Among Incarcerated People in California State Prisons: Retrospective Cohort Study
Abstract
Background: Prisons and jails are high-risk settings for coronavirus disease 2019 (COVID-19). Vaccines may substantially reduce these risks, but evidence is needed on COVID-19 vaccine effectiveness for incarcerated people, who are confined in large, risky congregate settings.
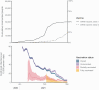
Methods: We conducted a retrospective cohort study to estimate effectiveness of messenger RNA (mRNA) vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), against confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections among incarcerated people in California prisons from 22 December 2020 through 1 March 2021. The California Department of Corrections and Rehabilitation provided daily data for all prison residents including demographic, clinical, and carceral characteristics, as well as COVID-19 testing, vaccination, and outcomes. We estimated vaccine effectiveness using multivariable Cox models with time-varying covariates, adjusted for resident characteristics and infection rates across prisons.
Results: Among 60 707 cohort members, 49% received at least 1 BNT162b2 or mRNA-1273 dose during the study period. Estimated vaccine effectiveness was 74% (95% confidence interval [CI], 64%-82%) from day 14 after first dose until receipt of second dose and 97% (95% CI, 88%-99%) from day 14 after second dose. Effectiveness was similar among the subset of residents who were medically vulnerable: 74% (95% CI, 62%-82%) and 92% (95% CI, 74%-98%) from 14 days after first and second doses, respectively.
Conclusions: Consistent with results from randomized trials and observational studies in other populations, mRNA vaccines were highly effective in preventing SARS-CoV-2 infections among incarcerated people. Prioritizing incarcerated people for vaccination, redoubling efforts to boost vaccination, and continuing other ongoing mitigation practices are essential in preventing COVID-19 in this disproportionately affected population.
Keywords: COVID-19; SARS-CoV-2; effectiveness; vaccination; vaccine.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Update of
-
Effectiveness of COVID-19 Vaccines among Incarcerated People in California State Prisons: A Retrospective Cohort Study.medRxiv [Preprint]. 2021 Aug 18:2021.08.16.21262149. doi: 10.1101/2021.08.16.21262149. medRxiv. 2021. Update in: Clin Infect Dis. 2022 Aug 24;75(1):e838-e845. doi: 10.1093/cid/ciab1032. PMID: 34426814 Free PMC article. Updated. Preprint.
Comment in
-
Vaccination in Prisons and Jails: Corrections Needed in Future Plans.Clin Infect Dis. 2022 Aug 24;75(1):e846-e848. doi: 10.1093/cid/ciab1031. Clin Infect Dis. 2022. PMID: 35083486 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

