Offset analgesia is associated with opposing modulation of medial versus dorsolateral prefrontal cortex activations: A functional near-infrared spectroscopy study
- PMID: 35083928
- PMCID: PMC9047820
- DOI: 10.1177/17448069221074991
Offset analgesia is associated with opposing modulation of medial versus dorsolateral prefrontal cortex activations: A functional near-infrared spectroscopy study
Abstract
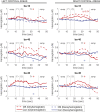
Offset analgesia is defined by a dramatic drop in perceived pain intensity with a relatively small decrease in noxious input. Although functional magnetic resonance imaging studies implicate subcortical descending inhibitory circuits during offset analgesia, the role of cortical areas remains unclear. The current study identifies cortical correlates of offset analgesia using functional near infrared spectroscopy (fNIRS). Twenty-four healthy volunteers underwent fNIRS scanning during offset (OS) and control (Con) heat stimuli applied to the forearm. After controlling for non-neural hemodynamic responses in superficial tissues, widespread increases in cortical oxygenated hemoglobin concentration were observed, reflecting cortical activation during heat pain. OS-Con contrasts revealed deactivations in bilateral medial prefrontal cortex (mPFC) and bilateral somatosensory cortex (SSC) associated with offset analgesia. Right dorsolateral prefrontal cortex (dlPFC) showed activation only during OS. These data demonstrate opposing cortical activation patterns during offset analgesia and support a model in which right dlPFC underlies ongoing evaluation of pain intensity change. With predictions of decreasing pain intensity, right dlPFC activation likely inhibits ascending noxious input via subcortical pathways resulting in SSC and mPFC deactivation. This study identifies cortical circuitry underlying offset analgesia and introduces the use of fNIRS to study pain modulation in an outpatient clinical environment.
Keywords: Offset analgesia; descending inhibition; endogenous analgesia; functional near-infrared spectroscopy; heat pain; human; pain.
Conflict of interest statement
Figures






References
-
- Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain 2019; 160: 1920–1932. DOI: 10.1097/j.pain.0000000000001590. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

