A Perspective on Late-Stage Aromatic C-H Bond Functionalization
- PMID: 35084173
- PMCID: PMC8855345
- DOI: 10.1021/jacs.1c10783
A Perspective on Late-Stage Aromatic C-H Bond Functionalization
Abstract
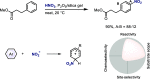
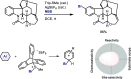
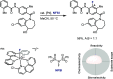
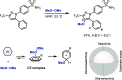
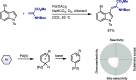
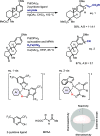
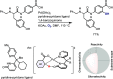
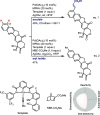
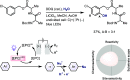
Late-stage functionalization of C-H bonds (C-H LSF) can provide a straightforward approach to the efficient synthesis of functionalized complex molecules. However, C-H LSF is challenging because the C-H bond must be functionalized in the presence of various other functional groups. In this Perspective, we evaluate aromatic C-H LSF on the basis of four criteria─reactivity, chemoselectivity, site-selectivity, and substrate scope─and provide our own views on current challenges as well as promising strategies and areas of growth going forward.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
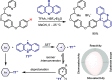
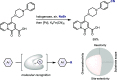
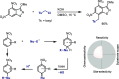
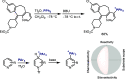
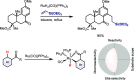
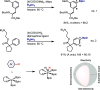
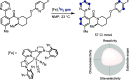
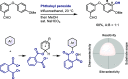
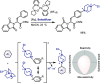
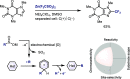
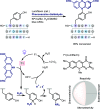
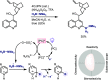

References
-
-
For reviews and perspectives on late-stage functionalization, see:
- Wencel-Delord J.; Glorius F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 2013, 5, 369–375. 10.1038/nchem.1607. - DOI - PubMed
- Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. 10.1039/C5CS00628G. - DOI - PubMed
- Börgel J.; Ritter T. Late-stage functionalization. Chem 2020, 6, 1877–1887. 10.1016/j.chempr.2020.07.007. - DOI
- Guillemard L.; Kaplaneris N.; Ackermann L.; Johansson M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021, 5, 522–545. 10.1038/s41570-021-00300-6. - DOI - PubMed
-
-
-
For reviews and books on drug structure–activity relationship studies, see:
- Trager W. F.Principles of Drug Metabolism 1: Redox Reactions. In Comprehensive Medicinal Chemistry II; Taylor J. B., Triggle D. J., Eds.; Elsevier: Oxford, 2007; pp 87–132.
- Beale J. M.; Block J. H.. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, 2011.
- Genovino J.; Sames D.; Hamann L. G.; Touré B. B. Accessing Drug Metabolites via Transition-Metal Catalyzed C–H Oxidation: The Liver as Synthetic Inspiration. Angew. Chem., Int. Ed. 2016, 55, 14218–14238. 10.1002/anie.201602644. - DOI - PubMed
-
-
-
For reviews on the effect of the fluorine substituent in drugs, see:
- Müller K.; Faeh C.; Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. - DOI - PubMed
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; Del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. - DOI - PubMed
-
-
-
For reviews on PET study, see:
- Ametamey S. M.; Honer M.; Schubiger P. A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. - DOI - PubMed
- Matthews P. M.; Rabiner E. A.; Passchier J.; Gunn R. N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 2012, 73, 175–186. 10.1111/j.1365-2125.2011.04085.x. - DOI - PMC - PubMed
-
-
-
For reviews on the application of tritiated drugs, see:
- Voges R.; Heys J. R.; Moenius T.. Preparation of tritium-labeled compounds by chemical synthesis. Preparation of Compounds Labeled with Tritium and Carbon-14; John Wiley & Sons, 2009; pp 109–209.
- Isin E. M.; Elmore C. S.; Nilsson G. N.; Thompson R. A.; Weidolf L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem. Res. Toxicol. 2012, 25, 532–542. 10.1021/tx2005212. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

