Genome diversity of domesticated Acinetobacter baumannii ATCC 19606T strains
- PMID: 35084299
- PMCID: PMC8914354
- DOI: 10.1099/mgen.0.000749
Genome diversity of domesticated Acinetobacter baumannii ATCC 19606T strains
Abstract
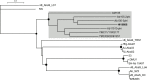
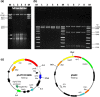
Acinetobacter baumannii has emerged as an important opportunistic pathogen worldwide, being responsible for large outbreaks for nosocomial infections, primarily in intensive care units. A. baumannii ATCC 19606T is the species type strain, and a reference organism in many laboratories due to its low virulence, amenability to genetic manipulation and extensive antibiotic susceptibility. We wondered if frequent propagation of A. baumannii ATCC 19606T in different laboratories may have driven micro- and macro-evolutionary events that could determine inter-laboratory differences of genome-based data. By combining Illumina MiSeq, MinION and Sanger technologies, we generated a high-quality whole-genome sequence of A. baumannii ATCC 19606T, then performed a comparative genome analysis between A. baumannii ATCC 19606T strains from several research laboratories and a reference collection. Differences between publicly available ATCC 19606T genome sequences were observed, including SNPs, macro- and micro-deletions, and the uneven presence of a 52 kb prophage belonging to genus Vieuvirus. Two plasmids, pMAC and p1ATCC19606, were invariably detected in all tested strains. The presence of a putative replicase, a replication origin containing four 22-mer direct repeats, and a toxin-antitoxin system implicated in plasmid stability were predicted by in silico analysis of p1ATCC19606, and experimentally confirmed. This work refines the sequence, structure and functional annotation of the A. baumannii ATCC 19606T genome, and highlights some remarkable differences between domesticated strains, likely resulting from genetic drift.
Keywords: Acinetobacter baumannii ATCC 19606T; genome refinement; native plasmids; strain domestication; Φ19606 phage.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

