Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK
- PMID: 35084596
- PMCID: PMC8795249
- DOI: 10.1186/s13568-022-01348-3
Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK
Abstract
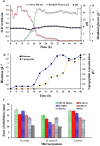
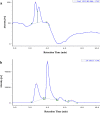
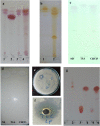
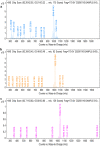
Bacillus velezensis SK having broad-spectrum antimicrobial activity has been isolated from soil. The efficient extraction of antimicrobial compounds produced in various mediums has been done using Diaion HP-20 resin. Further, characterization of an antimicrobial compound by TLC, FTIR, in-situ bioautography analysis revealed the presence of cyclic lipopeptides, which is then purified by the combination of silica gel, size exclusion, dual gradient, and RP-HPLC chromatography techniques. Growth kinetic studies showed that Bacillus velezensis SK produces a mixture of lipopeptides (1.33 gL-1). The lipopeptide exhibits good pH (2-10) and temperature stability up to 80 °C. LC-ESI-MS analysis of partially purified lipopeptide identified variant of surfactin, further analysis of purified chromatographic fractions revealed the occurrence of most abundant C15-surfactin homologues (m/z 1036.72 Da). The isolated surfactin exhibits good antimicrobial activity (1600 AU/ml) against drug-resistant food-born B. cereus and human pathogen Staphylococcus aureus. Hence, identified strain B. velezensis SK and its potent antibacterial surfactin lipopeptide could be used in various food and biomedical applications.
Keywords: Antibiotic-resistance; Antimicrobial peptides; B. velezensis SK; LC–ESI–MS; Lipopeptide; RP-HPLC.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that no competing interests exist.
Figures



References
-
- Alekshun MN, Levy SB. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell. 2007;128(6):1037–1050. - PubMed
-
- Balan SS, Kumar CG, Jayalakshmi S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol Res. 2017;194:1–9. - PubMed
-
- Chen L, Wang N, Wang X, Hu J, Wang S. Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour Technol. 2010;101(22):8822–8827. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

