Paramagnetic Chemical Probes for Studying Biological Macromolecules
- PMID: 35084831
- PMCID: PMC9136935
- DOI: 10.1021/acs.chemrev.1c00708
Paramagnetic Chemical Probes for Studying Biological Macromolecules
Abstract
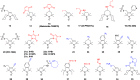
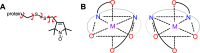
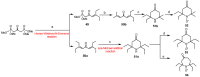
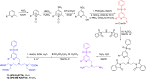
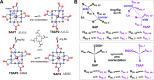
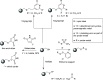
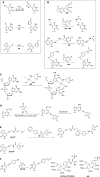
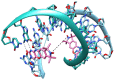
Paramagnetic chemical probes have been used in electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR) spectroscopy for more than four decades. Recent years witnessed a great increase in the variety of probes for the study of biological macromolecules (proteins, nucleic acids, and oligosaccharides). This Review aims to provide a comprehensive overview of the existing paramagnetic chemical probes, including chemical synthetic approaches, functional properties, and selected applications. Recent developments have seen, in particular, a rapid expansion of the range of lanthanoid probes with anisotropic magnetic susceptibilities for the generation of structural restraints based on residual dipolar couplings and pseudocontact shifts in solution and solid state NMR spectroscopy, mostly for protein studies. Also many new isotropic paramagnetic probes, suitable for NMR measurements of paramagnetic relaxation enhancements, as well as EPR spectroscopic studies (in particular double resonance techniques) have been developed and employed to investigate biological macromolecules. Notwithstanding the large number of reported probes, only few have found broad application and further development of probes for dedicated applications is foreseen.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

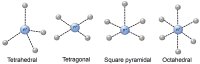
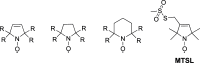

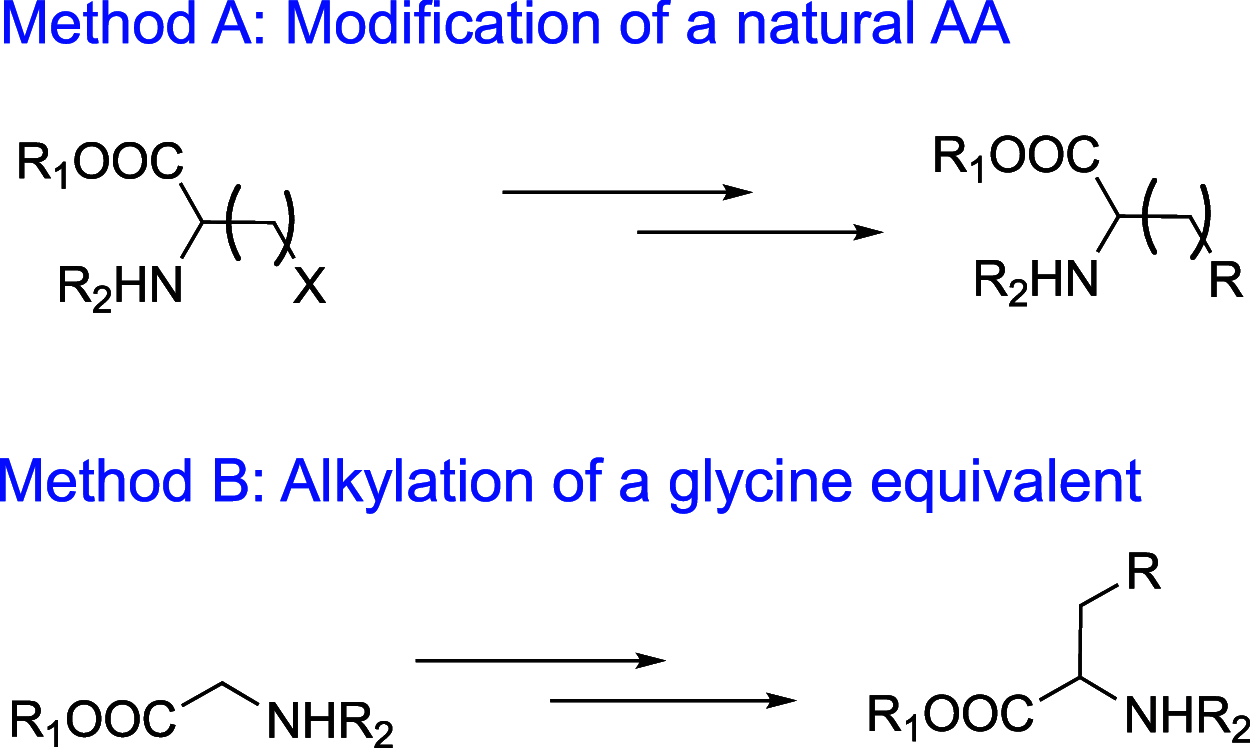

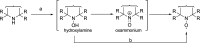

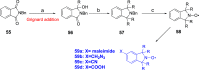








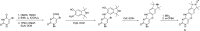








References
-
- Overhauser A. W. Polarization of Nuclei in Metals. Phys. Rev. 1953, 92, 411–415. 10.1103/PhysRev.92.411. - DOI
-
- Brüschweiler R.; Case D. A. Charaterization of Biomolecular Structure and Dynamics by NMR Cross Relaxation. Prog. Nucl. Magn. Reson. Spectrosc. 1994, 26, 27–58. 10.1016/0079-6565(94)80003-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

