High-dose rifampin improves bactericidal activity without increased intracerebral inflammation in animal models of tuberculous meningitis
- PMID: 35085105
- PMCID: PMC8920328
- DOI: 10.1172/JCI155851
High-dose rifampin improves bactericidal activity without increased intracerebral inflammation in animal models of tuberculous meningitis
Abstract
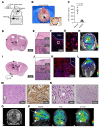
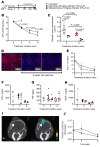
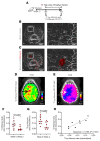
Tuberculous meningitis (TB meningitis) is the most severe form of tuberculosis (TB), requiring 12 months of multidrug treatment for cure, and is associated with high morbidity and mortality. High-dose rifampin (35 mg/kg/d) is safe and improves the bactericidal activity of the standard-dose (10 mg/kg/d) rifampin-containing TB regimen in pulmonary TB. However, there are conflicting clinical data regarding its benefit for TB meningitis, where outcomes may also be associated with intracerebral inflammation. We conducted cross-species studies in mice and rabbits, demonstrating that an intensified high-dose rifampin-containing regimen has significantly improved bactericidal activity for TB meningitis over the first-line, standard-dose rifampin regimen, without an increase in intracerebral inflammation. Positron emission tomography in live animals demonstrated spatially compartmentalized, lesion-specific pathology, with postmortem analyses showing discordant brain tissue and cerebrospinal fluid rifampin levels and inflammatory markers. Longitudinal multimodal imaging in the same cohort of animals during TB treatment as well as imaging studies in two cohorts of TB patients demonstrated that spatiotemporal changes in localized blood-brain barrier disruption in TB meningitis are an important driver of rifampin brain exposure. These data provide unique insights into the mechanisms underlying high-dose rifampin in TB meningitis with important implications for developing new antibiotic treatments for infections.
Keywords: Infectious disease; Microbiology; Neuroimaging; Neurological disorders; Pharmacology.
Figures

Similar articles
-
Linezolid does not improve bactericidal activity of rifampin-containing first-line regimens in animal models of TB meningitis.Int J Antimicrob Agents. 2024 Jan;63(1):107048. doi: 10.1016/j.ijantimicag.2023.107048. Epub 2023 Dec 5. Int J Antimicrob Agents. 2024. PMID: 38061419 Free PMC article.
-
Intensified treatment with high dose rifampicin and levofloxacin compared to standard treatment for adult patients with tuberculous meningitis (TBM-IT): protocol for a randomized controlled trial.Trials. 2011 Feb 2;12:25. doi: 10.1186/1745-6215-12-25. Trials. 2011. PMID: 21288325 Free PMC article. Clinical Trial.
-
Dynamic PET reveals compartmentalized brain and lung tissue antibiotic exposures of tuberculosis drugs.Nat Commun. 2024 Aug 14;15(1):6657. doi: 10.1038/s41467-024-50989-4. Nat Commun. 2024. PMID: 39143055 Free PMC article.
-
Tuberculous meningitis.Handb Clin Neurol. 2013;112:1135-8. doi: 10.1016/B978-0-444-52910-7.00033-7. Handb Clin Neurol. 2013. PMID: 23622321 Review.
-
Fluoroquinolones in the management of tuberculous meningitis: Systematic review and meta-analysis.J Infect. 2018 Oct;77(4):261-275. doi: 10.1016/j.jinf.2018.06.009. Epub 2018 Jul 12. J Infect. 2018. PMID: 30017610
Cited by
-
Pharmacokinetic study of high-dose oral rifampicin in critically Ill patients with multidrug-resistant Acinetobacter baumannii infection.Daru. 2022 Dec;30(2):311-322. doi: 10.1007/s40199-022-00449-5. Epub 2022 Sep 7. Daru. 2022. PMID: 36069988 Free PMC article.
-
Commonly Used Therapeutics Associated with Changes in Arousal Inhibit GABAAR Activation.Biomolecules. 2023 Feb 15;13(2):365. doi: 10.3390/biom13020365. Biomolecules. 2023. PMID: 36830736 Free PMC article.
-
Dynamic PET Reveals Compartmentalized Brain and Lung Tissue Antibiotic Exposures.Res Sq [Preprint]. 2024 Mar 21:rs.3.rs-4096014. doi: 10.21203/rs.3.rs-4096014/v1. Res Sq. 2024. Update in: Nat Commun. 2024 Aug 14;15(1):6657. doi: 10.1038/s41467-024-50989-4. PMID: 38562706 Free PMC article. Updated. Preprint.
-
The Development and Validation of Radiopharmaceuticals Targeting Bacterial Infection.J Nucl Med. 2023 Nov;64(11):1676-1682. doi: 10.2967/jnumed.123.265906. Epub 2023 Sep 28. J Nucl Med. 2023. PMID: 37770110 Free PMC article.
-
Current Insights into Diagnosing and Treating Neurotuberculosis in Adults.CNS Drugs. 2023 Nov;37(11):957-972. doi: 10.1007/s40263-023-01047-y. Epub 2023 Nov 17. CNS Drugs. 2023. PMID: 37978095 Review.

