Sterilized human skin graft with a dose of 25 kGy provides a privileged immune and collagen microenvironment in the adhesion of Nude mice wounds
- PMID: 35085314
- PMCID: PMC8794154
- DOI: 10.1371/journal.pone.0262532
Sterilized human skin graft with a dose of 25 kGy provides a privileged immune and collagen microenvironment in the adhesion of Nude mice wounds
Abstract
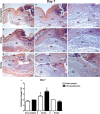
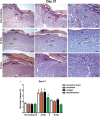
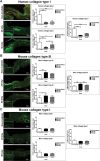
This study aimed to report the effects of different doses of ionizing radiation on inflammatory and repair stage of human skin graft adherence in Nude mice wounds. Animals were divided into transplanted with irradiated human skin grafts (IHSG) at 25 and 50 kGy (IHSG 25 kGy; IHSG 50 kGy) and non-IHSG and euthanized on the 3rd, 7th and 21st days after the surgery, by gross and microscopic changes, immunostaining for human type I collagen (Col I) and mouse Col I and Col III and inflammatory cells. We found an effectiveness of human split-thickness graft adherence in mice transplanted with IHSG 25 kGy, as well decrease in dermo-epidermal necrosis and neutrophils, lower loss of skin thickness, epithelization and neo-vascularization. Day 21 post-transplantation with IHSG 25 kGy was observed a well-preserved human skin in the border of the graft, a prominent granulation tissue in an organization by proliferated fibroblasts, Col III deposition and increased B-cells and macrophages. A complete adherence of human skin graft occurred with IHSG 25 kGy. We suggest that the ionizing radiation at 25 kGy mediates inflammation and the repair stage of human skin graft adherence in murine model, thus emerging as a potential tool in healing cutaneous wounds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kellar RS, Diller RB, Tabor AJ, Dominguez DD, Audet RG, Bardsley TA, et al.. Improved wound closure rates and mechanical properties resembling native skin in murine diabetic wounds treated with a tropoelastin and collagen wound healing device. J diabetes Clin Res. 2020;2(3): 86–99. doi: 10.33696/diabetes.1.024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

