Clinical Characteristics and Outcomes of Patients With COVID-19-Associated Acute Respiratory Distress Syndrome Who Underwent Lung Transplant
- PMID: 35085383
- PMCID: PMC8796055
- DOI: 10.1001/jama.2022.0204
Clinical Characteristics and Outcomes of Patients With COVID-19-Associated Acute Respiratory Distress Syndrome Who Underwent Lung Transplant
Abstract
Importance: Lung transplantation is a potentially lifesaving treatment for patients who are critically ill due to COVID-19-associated acute respiratory distress syndrome (ARDS), but there is limited information about the long-term outcome.
Objective: To report the clinical characteristics and outcomes of patients who had COVID-19-associated ARDS and underwent a lung transplant at a single US hospital.
Design, setting, and participants: Retrospective case series of 102 consecutive patients who underwent a lung transplant at Northwestern University Medical Center in Chicago, Illinois, between January 21, 2020, and September 30, 2021, including 30 patients who had COVID-19-associated ARDS. The date of final follow-up was November 15, 2021.
Exposures: Lung transplant.
Main outcomes and measures: Demographic, clinical, laboratory, and treatment data were collected and analyzed. Outcomes of lung transplant, including postoperative complications, intensive care unit and hospital length of stay, and survival, were recorded.
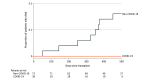
Results: Among the 102 lung transplant recipients, 30 patients (median age, 53 years [range, 27 to 62]; 13 women [43%]) had COVID-19-associated ARDS and 72 patients (median age, 62 years [range, 22 to 74]; 32 women [44%]) had chronic end-stage lung disease without COVID-19. For lung transplant recipients with COVID-19 compared with those without COVID-19, the median lung allocation scores were 85.8 vs 46.7, the median time on the lung transplant waitlist was 11.5 vs 15 days, and preoperative venovenous extracorporeal membrane oxygenation (ECMO) was used in 56.7% vs 1.4%, respectively. During transplant, patients who had COVID-19-associated ARDS received transfusion of a median of 6.5 units of packed red blood cells vs 0 in those without COVID-19, 96.7% vs 62.5% underwent intraoperative venoarterial ECMO, and the median operative time was 8.5 vs 7.4 hours, respectively. Postoperatively, the rates of primary graft dysfunction (grades 1 to 3) within 72 hours were 70% in the COVID-19 cohort vs 20.8% in those without COVID-19, the median time receiving invasive mechanical ventilation was 6.5 vs 2.0 days, the median duration of intensive care unit stay was 18 vs 9 days, the median post-lung transplant hospitalization duration was 28.5 vs 16 days, and 13.3% vs 5.5% required permanent hemodialysis, respectively. None of the lung transplant recipients who had COVID-19-associated ARDS demonstrated antibody-mediated rejection compared with 12.5% in those without COVID-19. At follow-up, all 30 lung transplant recipients who had COVID-19-associated ARDS were alive (median follow-up, 351 days [IQR, 176-555] after transplant) vs 60 patients (83%) who were alive in the non-COVID-19 cohort (median follow-up, 488 days [IQR, 368-570] after lung transplant).
Conclusions and relevance: In this single-center case series of 102 consecutive patients who underwent a lung transplant between January 21, 2020, and September 30, 2021, survival was 100% in the 30 patients who had COVID-19-associated ARDS as of November 15, 2021.
Conflict of interest statement
Figures
Comment in
-
Characteristics and Outcomes of Patients With COVID-19-Associated ARDS Who Underwent Lung Transplant.JAMA. 2022 Jun 28;327(24):2454. doi: 10.1001/jama.2022.6634. JAMA. 2022. PMID: 35763004 No abstract available.
References
-
- Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

