Detection of SARS-CoV-2 variants by Abbott molecular, antigen, and serological tests
- PMID: 35086043
- PMCID: PMC8770247
- DOI: 10.1016/j.jcv.2022.105080
Detection of SARS-CoV-2 variants by Abbott molecular, antigen, and serological tests
Abstract
Background: Viral diversity presents an ongoing challenge for diagnostic tests, which need to accurately detect all circulating variants. The Abbott Global Surveillance program monitors severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants and their impact on diagnostic test performance.
Objectives: To evaluate the capacity of Abbott molecular, antigen, and serologic assays to detect circulating SARS-CoV-2 variants, including all current variants of concern (VOC): B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma) and B.1.617.2 (delta).
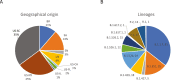
Study design: Dilutions of variant virus cultures (B.1.1.7, B.1.351, B.1.429, B.1.526.1, B.1.526.2, B.1.617.1, B.1.617.2, P.1, R.1 and control isolate WA1) and a panel of N = 248 clinical samples from patients with sequence confirmed variant infections (B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, B.1.526.1, B.1.526.2, P.1, P.2, R.1) were evaluated on at least one assay: Abbott ID NOW COVID-19, m2000 RealTime SARS-CoV-2, Alinity m SARS-CoV-2, and Alinity m Resp-4-Plex molecular assays; the BinaxNOW COVID-19 Ag Card and Panbio COVID-19 Ag Rapid Test Device; and the ARCHITECT/Alinity i SARS-CoV-2 IgG and AdviseDx IgM assays, Panbio COVID-19 IgG assay, and ARCHITECT/Alinity i AdviseDx SARS-CoV-2 IgG II assay.
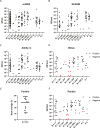
Results: Consistent with in silico predictions, each molecular and antigen assay detected VOC virus cultures with equivalent sensitivity to the WA1 control strain. Notably, 100% of all tested variant patient specimens were detected by molecular assays (N = 197 m2000, N = 88 Alinity m, N = 99 ID NOW), and lateral flow assays had a sensitivity of >94% for specimens with genome equivalents (GE) per device above 4 log (85/88, Panbio; 54/57 Binax). Furthermore, Abbott antibody assays detected IgG and IgM in 94-100% of sera from immune competent B.1.1.7 patients 15-26 days after symptom onset.
Conclusions: These data confirm variant detection for 11 SARS-CoV-2 assays, which is consistent with each assay target region being highly conserved. Importantly, alpha, beta, gamma, and delta VOCs were detected by molecular and antigen assays, indicating that these tests may be suitable for widescale use where VOCs predominate.
Keywords: Antibody assays; Molecular diagnostics; Rapid antigen tests; SARS-CoV-2; Variant of concern.
Copyright © 2022 Abbott Laboratories. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
MAR, AO, BH, CL, XL, MGB, TVM, AM, GSO, and GC are employees and shareholders of Abbott Laboratories.This work was funded by Abbott Laboratories.
Figures
References
-
- Investigation of novel SARS-COV-2 variant Variant of concern 202012/01. Public Health England. 2020
-
- Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. MedRxiv. 2021
-
- Tada T., Dcosta B.M., Samanovic-Golden M., Herati R.S., Cornelius A., Mulligan M.J., et al. Neutralization of viruses with European, South African, and United States SARS-CoV-2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine-elicited antibodies. bioRxiv. 2021
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

