Bioprinted microvasculature: progressing from structure to function
- PMID: 35086069
- PMCID: PMC8988885
- DOI: 10.1088/1758-5090/ac4fb5
Bioprinted microvasculature: progressing from structure to function
Abstract
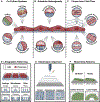
Three-dimensional (3D) bioprinting seeks to unlock the rapid generation of complex tissue constructs, but long-standing challenges with efficientin vitromicrovascularization must be solved before this can become a reality. Microvasculature is particularly challenging to biofabricate due to the presence of a hollow lumen, a hierarchically branched network topology, and a complex signaling milieu. All of these characteristics are required for proper microvascular-and, thus, tissue-function. While several techniques have been developed to address distinct portions of this microvascularization challenge, no single approach is capable of simultaneously recreating all three microvascular characteristics. In this review, we present a three-part framework that proposes integration of existing techniques to generate mature microvascular constructs. First, extrusion-based 3D bioprinting creates a mesoscale foundation of hollow, endothelialized channels. Second, biochemical and biophysical cues induce endothelial sprouting to create a capillary-mimetic network. Third, the construct is conditioned to enhance network maturity. Across all three of these stages, we highlight the potential for extrusion-based bioprinting to become a central technique for engineering hierarchical microvasculature. We envision that the successful biofabrication of functionally engineered microvasculature will address a critical need in tissue engineering, and propel further advances in regenerative medicine andex vivohuman tissue modeling.
Keywords: 3D printing; endothelial sprouting; extrusion-based bioprinting; microvasculature; vascular function; vascular structure.
© 2022 IOP Publishing Ltd.
Figures



References
-
- Fitzsimmons RE, Aquilino MS, Quigley J, Chebotarev O, Tarlan F and Simmons CA Generating vascular channels within hydrogel constructs using an economical open-source 3D bioprinter and thermoreversible gels. Bioprinting 9, 7–18 (2018). doi:10.1016/j.bprint.2018.02.001 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
