Risk profiling of patients with relapsed/refractory diffuse large B-cell lymphoma by measuring circulating tumor DNA
- PMID: 35086141
- PMCID: PMC8941482
- DOI: 10.1182/bloodadvances.2021006415
Risk profiling of patients with relapsed/refractory diffuse large B-cell lymphoma by measuring circulating tumor DNA
Abstract
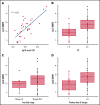
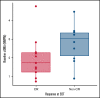
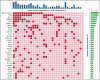
Patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) have heterogeneous outcomes; durable remissions are infrequently observed with standard approaches. Circulating tumor DNA (ctDNA) assessment is a sensitive, potentially prognostic tool in this setting. We assessed baseline ctDNA to identify patients with R/R DLBCL at high risk of relapse after receiving polatuzumab vedotin and bendamustine plus rituximab (BR) or BR alone. Patients were transplant ineligible and had received ≥1 prior line of therapy. The ctDNA assay, based on a customized panel of recurrently mutated genes in DLBCL, measured mutant molecules per mL (MMPM) at baseline and end of treatment (EOT). Endpoints included progression-free survival (PFS) and overall survival (OS) in subgroups stratified by baseline ctDNA and log-fold change in ctDNA at EOT vs baseline. In biomarker-evaluable patients (n = 33), baseline ctDNA level correlated with serum lactate dehydrogenase (LDH) concentration, number of prior therapies, stage, and International Prognostic Index (IPI). After adjusting for number of prior therapies ≥2, IPI score ≥3, and LDH above the upper limit of normal, high (greater than median) baseline ctDNA MMPM was independently prognostic for shorter PFS (adjusted hazard ratio [HR], 0.18 [95% CI, 0.05-0.65]) and OS (adjusted HR, 0.20 [95% CI, 0.06-0.68]). In 23 patients with baseline and EOT samples, a significantly greater decrease in ctDNA MMPM was observed in patients with complete response (CR) (n = 13) than those without CR (n = 10); P = .0025. Baseline ctDNA assessment may identify patients at high risk of progression and should be further evaluated as a monitoring tool in R/R DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT02257567.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



References
-
- Polson AG, Calemine-Fenaux J, Chan P, et al. . Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection [published correction appears in Cancer Res. 2010;70(3):1275]. Cancer Res. 2009;69(6):2358-2364. - PubMed
-
- Okazaki M, Luo Y, Han T, Yoshida M, Seon BK. Three new monoclonal antibodies that define a unique antigen associated with prolymphocytic leukemia/non-Hodgkin’s lymphoma and are effectively internalized after binding to the cell surface antigen. Blood. 1993;81(1):84-94. - PubMed
-
- U.S. Food and Drug Administration. POLIVY U.S. Prescribing Information. https://wwwaccessdatafdagov/drugsatfda_docs/label/2019/761121s000lbl.pdf. Accessed 5 March 2021.
-
- EMA. POLIVY: product information. https://www.ema.europa.eu/en/documents/product-information/polivy-epar-p.... Accessed 5 March 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

