Ero1α-Dependent ERp44 Dissociation From RyR2 Contributes to Cardiac Arrhythmia
- PMID: 35086342
- PMCID: PMC8893133
- DOI: 10.1161/CIRCRESAHA.121.320531
Ero1α-Dependent ERp44 Dissociation From RyR2 Contributes to Cardiac Arrhythmia
Abstract
Background: Oxidative stress in cardiac disease promotes proarrhythmic disturbances in Ca2+ homeostasis, impairing luminal Ca2+ regulation of the sarcoplasmic reticulum (SR) Ca2+ release channel, the RyR2 (ryanodine receptor), and increasing channel activity. However, exact mechanisms underlying redox-mediated increase of RyR2 function in cardiac disease remain elusive. We tested whether the oxidoreductase family of proteins that dynamically regulate the oxidative environment within the SR are involved in this process.
Methods: A rat model of hypertrophy induced by thoracic aortic banding (TAB) was used for ex vivo whole heart optical mapping and for Ca2+ and reactive oxygen species imaging in isolated ventricular myocytes (VMs).
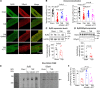
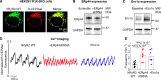
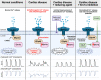
Results: The SR-targeted reactive oxygen species biosensor ERroGFP showed increased intra-SR oxidation in TAB VMs that was associated with increased expression of Ero1α (endoplasmic reticulum oxidoreductase 1 alpha). Pharmacological (EN460) or genetic Ero1α inhibition normalized SR redox state, increased Ca2+ transient amplitude and SR Ca2+ content, and reduced proarrhythmic spontaneous Ca2+ waves in TAB VMs under β-adrenergic stimulation (isoproterenol). Ero1α overexpression in Sham VMs had opposite effects. Ero1α inhibition attenuated Ca2+-dependent ventricular tachyarrhythmias in TAB hearts challenged with isoproterenol. Experiments in TAB VMs and human embryonic kidney 293 cells expressing human RyR2 revealed that an Ero1α-mediated increase in SR Ca2+-channel activity involves dissociation of intraluminal protein ERp44 (endoplasmic reticulum protein 44) from the RyR2 complex. Site-directed mutagenesis and molecular dynamics simulations demonstrated a novel redox-sensitive association of ERp44 with RyR2 mediated by intraluminal cysteine 4806. ERp44-RyR2 association in TAB VMs was restored by Ero1α inhibition, but not by reducing agent dithiothreitol, as hypo-oxidation precludes formation of covalent bond between RyR2 and ERp44.
Conclusions: A novel axis of intraluminal interaction between RyR2, ERp44, and Ero1α has been identified. Ero1α inhibition exhibits promising therapeutic potential by stabilizing RyR2-ERp44 complex, thereby reducing spontaneous Ca2+ release and Ca2+-dependent tachyarrhythmias in hypertrophic hearts, without causing hypo-oxidative stress in the SR.
Keywords: cardiovascular diseases; constriction; heart failure; homeostasis; oxidoreductases.
Figures





Comment in
-
Luminal Oxidative Regulation of the Ryanodine Receptor: More Sides to the Story?Circ Res. 2022 Mar 4;130(5):725-727. doi: 10.1161/CIRCRESAHA.122.320798. Epub 2022 Mar 3. Circ Res. 2022. PMID: 35239403 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

