Risk of Guillain-Barré syndrome after vaccination against human papillomavirus: a systematic review and meta-analysis, 1 January 2000 to 4 April 2020
- PMID: 35086611
- PMCID: PMC8796292
- DOI: 10.2807/1560-7917.ES.2022.27.4.2001619
Risk of Guillain-Barré syndrome after vaccination against human papillomavirus: a systematic review and meta-analysis, 1 January 2000 to 4 April 2020
Abstract
BackgroundGuillain-Barré syndrome (GBS) is a rare autoimmune disease that can follow viral infections and has in a few cases been linked to vaccinations. Pre-licensure clinical trials did not observe an association between human papillomavirus (HPV) vaccination and GBS, a post-marketing study from 2017 reported an increased relative risk.AimWe assessed the risk of GBS after HPV vaccination through a systematic literature review and meta-analysis.MethodsWe searched Embase, MEDLINE and Cochrane for studies reporting on the risk of GBS after HPV vaccination in individuals aged ≥ 9 years, published between 1 January 2000 and 4 April 2020, excluding studies without a comparator group. Seven studies reporting relative effect sizes were pooled using random-effects meta-analysis. We assessed quality of evidence using the GRADE approach. Study protocol was registered (PROSPERO No. #CRD42019123533).ResultsOf 602 identified records, we included 25 studies. Based on over 10 million reports, cases of GBS were rare. In 22 studies no increased risk was observed, while in three studies a signal of increased risk of GBS after HPV vaccination was identified. Meta-analysis yielded a pooled random-effects ratio of 1.21 (95% CI: 0.60-2.43); I2 = 72% (95% CI: 36-88). This translates to a number needed to harm of one million to be vaccinated to generate one GBS case. Quality of evidence was very low.ConclusionsThe absolute and relative risk of GBS after HPV vaccination is very low and lacks statistical significance. This is reassuring for the already implemented vaccination programmes and should be used in respective communication activities.
Keywords: Guillain-Barre syndrome; Papillomaviridae; Systematic Review; vaccination.
Conflict of interest statement
Figures
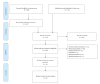
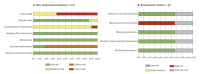

References
-
- Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily M-C, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497-509. 10.1016/S0140-6736(19)30298-3 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
