Early mortality during rifampicin-resistant TB treatment
- PMID: 35086627
- PMCID: PMC8802559
- DOI: 10.5588/ijtld.21.0494
Early mortality during rifampicin-resistant TB treatment
Abstract
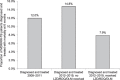
BACKGROUND: Data suggest that treatment with newer TB drugs (linezolid [LZD], bedaquiline [BDQ] and delamanid [DLM]), used in Khayelitsha, South Africa, since 2012, reduces mortality due to rifampicin-resistant TB (RR-TB).METHODS: This was a retrospective cohort study to assess 6-month mortality among RR-TB patients diagnosed between 2008 and 2019.RESULTS: By 6 months, 236/2,008 (12%) patients died; 12% (78/651) among those diagnosed in 2008-2011, and respectively 8% (49/619) and 15% (109/738) with and without LZD/BDQ/DLM in 2012-2019. Multivariable analysis showed a small, non-significant mortality reduction with LZD/BDQ/DLM use compared to the 2008-2011 period (aOR 0.79, 95% CI 0.5-1.2). Inpatient treatment initiation (aOR 3.2, 95% CI 2.4-4.4), fluoroquinolone (FQ) resistance (aOR 2.7, 95% CI 1.8-4.2) and female sex (aOR 1.5, 95% CI 1.1-2.0) were also associated with mortality. When restricted to 2012-2019, use of LZD/BDQ/DLM was associated with lower mortality (aOR 0.58, 95% CI 0.39-0.87).CONCLUSIONS: While LZD/BDQ/DLM reduced 6-month mortality between 2012 and 2019, there was no significant effect overall. These findings may be due to initially restricted LZD/BDQ/DLM use for those with high-level resistance or treatment failure. Additional contributors include increased treatment initiation among individuals who would have otherwise died before treatment due to universal drug susceptibility testing from 2012, an effect that also likely contributed to higher mortality among females (survival through to care-seeking).
CONTEXTE :: Certaines données suggèrent qu’un traitement à base des nouveaux médicaments antituberculeux (linézolide [LZD], bédaquiline [BDQ] et délamanide [DLM]), administré à Khayelitsha, Afrique du Sud, depuis 2012, réduit la mortalité due à la TB résistante à la rifampicine (RR-TB).
MÉTHODES :: Il s’agissait d’une étude de cohorte rétrospective visant à évaluer la mortalité à 6 mois chez des patients atteints de RR-TB diagnostiqués entre 2008 et 2019.
RÉSULTATS :: À 6 mois, 236/2 008 (12%) patients étaient décédés ; 12% (78/651) de ceux diagnostiqués en 2008–2011, et respectivement 8% (49/619) et 15% (109/738) avec et sans LZD/BDQ/DLM en 2012–2019. L’analyse multivariable a démontré une faible réduction non significative de la mortalité avec l’utilisation de LZD/BDQ/DLM par rapport à la période 2008–2011 (aOR 0,79 ; IC 95% 0,5–1,2). L’instauration du traitement chez les patients hospitalisés (aOR 3,2 ; IC 95% 2,4–4,4), la résistance aux fluoroquinolones (FQ) (aOR 2,7 ; IC 95% 1,8–4,2) et le genre féminin (aOR 1,5 ; IC 95% 1,1–2,0) étaient également associés à la mortalité. En restreignant l’analyse à la période 2012–2019, l’utilisation de LZD/BDQ/DLM était associée à une plus faible mortalité (aOR 0,58 ; IC 95% 0,39–0,87).
CONCLUSIONS :: Bien que LZD/BDQ/DLM aient réduit la mortalité à 6 mois entre 2012 et 2019, aucun effet global significatif n’a été observé. Ces résultats peuvent être dus à l’utilisation initiale restreinte de LZD/BDQ/DLM pour les patients présentant de forts taux de résistance ou en échec de traitement. Les facteurs contributifs supplémentaires comprennent une hausse des instaurations de traitement chez des patients qui seraient autrement décédés avant l’instauration en raison de la mise en place universelle des tests de sensibilité aux médicaments à partir de 2012. Cet effet a également probablement contribué à une plus grande mortalité chez les femmes (survie jusqu’à la recherche de soins).
Figures
References
-
- World Health Organization Geneva, Switzerland: WHO; 2020. Global tuberculosis report, 2020. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-en... Accessed January 2021.
-
- Ndjeka N, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc lung Dis. 2015;19(8):979–985. - PubMed
-
- Ndjeka N, et al. High treatment success rate for multidrugresistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J. 2018;52(6):1–2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

