The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation
- PMID: 35086828
- PMCID: PMC9520034
- DOI: 10.1183/13993003.03697-2020
The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation
Abstract
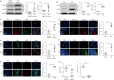
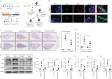
Although DNA methylation has been recognised in the pathogenesis of idiopathic pulmonary fibrosis (IPF), the exact mechanisms are yet to be fully addressed. Herein, we demonstrate that lungs originated from IPF patients and mice after bleomycin (BLM)-induced pulmonary fibrosis are characterised by altered DNA methylation along with overexpression in myofibroblasts of methyl-CpG-binding domain 2 (MBD2), a reader responsible for interpreting DNA methylome-encoded information. Specifically, depletion of Mbd2 in fibroblasts or myofibroblasts protected mice from BLM-induced pulmonary fibrosis coupled with a significant reduction of fibroblast differentiation. Mechanistically, transforming growth factor (TGF)-β1 induced a positive feedback regulatory loop between TGF-β receptor I (TβRI), Smad3 and Mbd2, and erythroid differentiation regulator 1 (Erdr1). TGF-β1 induced fibroblasts to undergo a global DNA hypermethylation along with Mbd2 overexpression in a TβRI/Smad3 dependent manner, and Mbd2 selectively bound to the methylated CpG DNA within the Erdr1 promoter to repress its expression, through which it enhanced TGF-β/Smad signalling to promote differentiation of fibroblast into myofibroblast and exacerbate pulmonary fibrosis. Therefore, enhancing Erdr1 expression strikingly reversed established pulmonary fibrosis. Collectively, our data support that strategies aimed at silencing Mbd2 or increasing Erdr1 could be viable therapeutic approaches for prevention and treatment of pulmonary fibrosis in clinical settings.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: The authors declare no competing financial interests.
Figures






Comment in
-
New insights into methylome alterations and consequences during myofibroblastic differentiation in pulmonary fibrosis.Eur Respir J. 2022 Sep 29;60(3):2201536. doi: 10.1183/13993003.01536-2022. Print 2022 Sep. Eur Respir J. 2022. PMID: 36175026 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
